Human Neuroepithelial Stem Cell Regional Specificity Enables Spinal Cord Repair Through a Relay Circuit
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intramedullary Cystic Lesions Ofthe Conus Medullaris
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.31.2.106 on 1 April 1968. Downloaded from J. Neurol. Neurosurg. Psychiat., 1968, 31, 106-109 Intramedullary cystic lesions of the conus medullaris SAMI I. NASSAR, JAMES W. CORRELL, AND EDGAR M. HOUSEPIAN From the Department of Neurosurgery, College ofPhysicians and Surgeons, Columbia University, and the Neurological Institute of the Columbia-Presbyterian Medical Center, New York, U.S.A. Intramedullary cystic lesions of the conus medullaris of the aetiology, these cysts may simulate the clinical are rare. Although an extensive literature describes picture of syringomyelia. syringomyelia as being a frequent basis for cystic The cases of cysts of the conus medullaris re- cervico-thoracic lesions it is apparent that this ported here simulated the clinical picture of does not occur frequently in the lumbosacral region syringomyelia, tumour, or lumbar disc disease. (Kirgis and Echols, 1949; Netsky, 1953; Rand and The radiographic findings in each case were inter- Rand, 1960; Love and Olafson, 1966). Poser (1956), preted as indicating the presence ofan intramedullary in a review of 234 cases of syringomyelia, found tumour. The correct diagnosis was made in each that the cavity extended into the lumbosacral region case only at operation. in only 12-6% and in only five cases were the Protected by copyright. cavities restricted to the lumbosacral segments. Some authors (Thevenard, 1942; Andre, 1951) CASE REPORTS question the occurrence of syringomyelia in the lower spinal cord. Nevertheless a high incidence CASE 1 (F.T., NO. 179 16 92) A 22-year-old negro male of constitutional defects has been noted among was admitted complaining of weakness and pain in the syringomyelia patients and members of their legs for three years. -

Split Spinal Cord Malformations in Children
Split spinal cord malformations in children Yusuf Ersahin, M.D., Saffet Mutluer, M.D., Sevgül Kocaman, R.N., and Eren Demirtas, M.D. Division of Pediatric Neurosurgery, Department of Neurosurgery, and Department of Pathology, Ege University Faculty of Medicine, Izmir, Turkey The authors reviewed and analyzed information on 74 patients with split spinal cord malformations (SSCMs) treated between January 1, 1980 and December 31, 1996 at their institution with the aim of defining and classifying the malformations according to the method of Pang, et al. Computerized tomography myelography was superior to other radiological tools in defining the type of SSCM. There were 46 girls (62%) and 28 boys (38%) ranging in age from less than 1 day to 12 years (mean 33.08 months). The mean age (43.2 months) of the patients who exhibited neurological deficits and orthopedic deformities was significantly older than those (8.2 months) without deficits (p = 0.003). Fifty-two patients had a single Type I and 18 patients a single Type II SSCM; four patients had composite SSCMs. Sixty-two patients had at least one associated spinal lesion that could lead to spinal cord tethering. After surgery, the majority of the patients remained stable and clinical improvement was observed in 18 patients. The classification of SSCMs proposed by Pang, et al., will eliminate the current chaos in terminology. In all SSCMs, either a rigid or a fibrous septum was found to transfix the spinal cord. There was at least one unrelated lesion that caused tethering of the spinal cord in 85% of the patients. -

The Act of Controlling Adult Stem Cell Dynamics: Insights from Animal Models
biomolecules Review The Act of Controlling Adult Stem Cell Dynamics: Insights from Animal Models Meera Krishnan 1, Sahil Kumar 1, Luis Johnson Kangale 2,3 , Eric Ghigo 3,4 and Prasad Abnave 1,* 1 Regional Centre for Biotechnology, NCR Biotech Science Cluster, 3rd Milestone, Gurgaon-Faridabad Ex-pressway, Faridabad 121001, India; [email protected] (M.K.); [email protected] (S.K.) 2 IRD, AP-HM, SSA, VITROME, Aix-Marseille University, 13385 Marseille, France; [email protected] 3 Institut Hospitalo Universitaire Méditerranée Infection, 13385 Marseille, France; [email protected] 4 TechnoJouvence, 13385 Marseille, France * Correspondence: [email protected] Abstract: Adult stem cells (ASCs) are the undifferentiated cells that possess self-renewal and differ- entiation abilities. They are present in all major organ systems of the body and are uniquely reserved there during development for tissue maintenance during homeostasis, injury, and infection. They do so by promptly modulating the dynamics of proliferation, differentiation, survival, and migration. Any imbalance in these processes may result in regeneration failure or developing cancer. Hence, the dynamics of these various behaviors of ASCs need to always be precisely controlled. Several genetic and epigenetic factors have been demonstrated to be involved in tightly regulating the proliferation, differentiation, and self-renewal of ASCs. Understanding these mechanisms is of great importance, given the role of stem cells in regenerative medicine. Investigations on various animal models have played a significant part in enriching our knowledge and giving In Vivo in-sight into such ASCs regulatory mechanisms. In this review, we have discussed the recent In Vivo studies demonstrating the role of various genetic factors in regulating dynamics of different ASCs viz. -

The Spinal Cord Is a Nerve Column That Passes Downward from Brain Into the Vertebral Canal
The spinal cord is a nerve column that passes downward from brain into the vertebral canal. Recall that it is part of the CNS. Spinal nerves extend to/from the spinal cord and are part of the PNS. Length = about 17 inches Start = foramen magnum End = tapers to point (conus medullaris) st nd and terminates 1 –2 lumbar (L1-L2) vertebra Contains 31 segments à gives rise to 31 pairs of spinal nerves Note cervical and lumbar enlargements. cauda equina (“horse’s tail”) –collection of spinal nerves at inferior end of vertebral column (nerves coming off end of spinal cord) Meninges- cushion and protected by same 3 layers as brain. Extend past end of cord into vertebral canal à spinal tap because no cord A cross-section of the spinal cord resembles a butterfly with its wings outspread (gray matter) surrounded by white matter. GRAY MATTER or “butterfly” = bundles of cell bodies Posterior (dorsal) horns=association or interneurons (incoming somatosensory information) Lateral horns=autonomic neurons Anterior (ventral) horns=cell bodies of motor neurons Central canal-found within gray matter and filled with CSF White Matter: 3 Regions: Posterior (dorsal) white column or funiculi – contains only ASCENDING tracts à sensory only Lateral white column or funiculi – both ascending and descending tracts à sensory and motor Anterior (ventral) white column or funiculi – both ascending and descending tracts à sensory and motor All nerve tracts made of mylinated axons with same destination and function Associated Structures: Dorsal Roots = made -

Regulation of Adult Neurogenesis in Mammalian Brain
International Journal of Molecular Sciences Review Regulation of Adult Neurogenesis in Mammalian Brain 1,2, 3, 3,4 Maria Victoria Niklison-Chirou y, Massimiliano Agostini y, Ivano Amelio and Gerry Melino 3,* 1 Centre for Therapeutic Innovation (CTI-Bath), Department of Pharmacy & Pharmacology, University of Bath, Bath BA2 7AY, UK; [email protected] 2 Blizard Institute of Cell and Molecular Science, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London E1 2AT, UK 3 Department of Experimental Medicine, TOR, University of Rome “Tor Vergata”, 00133 Rome, Italy; [email protected] (M.A.); [email protected] (I.A.) 4 School of Life Sciences, University of Nottingham, Nottingham NG7 2HU, UK * Correspondence: [email protected] These authors contributed equally to this work. y Received: 18 May 2020; Accepted: 7 July 2020; Published: 9 July 2020 Abstract: Adult neurogenesis is a multistage process by which neurons are generated and integrated into existing neuronal circuits. In the adult brain, neurogenesis is mainly localized in two specialized niches, the subgranular zone (SGZ) of the dentate gyrus and the subventricular zone (SVZ) adjacent to the lateral ventricles. Neurogenesis plays a fundamental role in postnatal brain, where it is required for neuronal plasticity. Moreover, perturbation of adult neurogenesis contributes to several human diseases, including cognitive impairment and neurodegenerative diseases. The interplay between extrinsic and intrinsic factors is fundamental in regulating neurogenesis. Over the past decades, several studies on intrinsic pathways, including transcription factors, have highlighted their fundamental role in regulating every stage of neurogenesis. However, it is likely that transcriptional regulation is part of a more sophisticated regulatory network, which includes epigenetic modifications, non-coding RNAs and metabolic pathways. -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

Rapid and Efficient Generation of Oligodendrocytes from Human
Rapid and efficient generation of oligodendrocytes PNAS PLUS from human induced pluripotent stem cells using transcription factors Marc Ehrlicha,b, Sabah Mozafaric,d,e,f, Michael Glatzab, Laura Starosta,b, Sergiy Velychkob, Anna-Lena Hallmanna,b, Qiao-Ling Cuig, Axel Schambachh, Kee-Pyo Kimb, Corinne Bachelinc,d,e,f, Antoine Marteync,d,e,f, Gunnar Hargusa,b, Radia Marie Johnsoni, Jack Antelg, Jared Sterneckertj, Holm Zaehresb,k, Hans R. Schölerb,l, Anne Baron-Van Evercoorenc,d,e,f, and Tanja Kuhlmanna,1 aInstitute of Neuropathology, University Hospital Münster, 48149 Muenster, Germany; bDepartment of Cell and Developmental Biology, Max Planck Institute for Molecular Biomedicine, 48149 Muenster, Germany; cINSERM, U1127, F-75013 Paris, France; dCNRS, UMR 7225, F-75013 Paris, France; eSorbonne Universités, Université Pierre et Marie Curie Paris 06, UM-75, F-75005 Paris, France; fInstitut du Cerveau et de la Moelle epinière-Groupe Hospitalier Pitié-Salpêtrière, F-75013 Paris, France; gMontreal Neurological Institute, McGill University, Montreal, QC, Canada H3A 2B4; hInstitute of Experimental Hematology, Hannover Medical School, 30625 Hannover, Germany; iDepartment of Physiology, McGill University, Montreal, QC, Canada H3A 2B4; jDFG Research Center for Regenerative Therapies, Technische Universität Dresden, 01307 Dresden, Germany; kMedical Faculty, Department of Anatomy and Molecular Embryology, Ruhr-University Bochum, 44801 Bochum, Germany; and lMedicial Faculty, Westphalian Wilhelms-University of Muenster, 48149 Muenster, Germany Edited by Brigid L. M. Hogan, Duke University Medical Center, Durham, NC, and approved February 1, 2017 (received for review August 30, 2016) Rapid and efficient protocols to generate oligodendrocytes (OL) more, these protocols require long culture periods (70–150 d) to + from human induced pluripotent stem cells (iPSC) are currently obtain O4 OL and show limited efficiency (9–12). -

The Conus Medullaris: a Comprehensive Review
THE SPINE SCHOLAR VOLUME 1, NUMBER 2, 2017 SEATTLE SCIENCE FOUNDATION REVIEW The Conus Medullaris: A Comprehensive Review Garrett Ng1, Anthony V. D’Antoni1, R. Shane Tubbs2 1 The CUNY School of Medicine, New York, NY 10031, USA 2 Department of Anatomical Sciences, St. George’s University, Grenada http:thespinescholar.com https:doi.org/10.26632/ss.10.2017.1.2 Key Words: anatomy, embryology, spinal cord, spine ABSTRACT The position of the conus medullaris within the vertebral canal varies. Given its role in sensory and motor function, a comprehensive understanding of the conus medullaris is necessary. PubMed and Google Scholar were used to review the literature on the conus medullaris. Pathological states and traumatic injury relating to the conus medullaris should be studied further. Spine Scholar 1:93-96, 2017 INTRODUCTION The conus medullaris (Fig. 1), also known as the medullary cone, is the distal end of the spinal cord. Its location varies, and in adults it tapers at approximately the first or second lumbar vertebra, ranging from T11 and L3 (Neel, 2016). Derived from the neural tube, the structure ascends in the vertebral canal because the growth rates of the spinal cord and the vertebral column differ during development (Salbacak et al., 2000). Figure 1: Schematic drawing of the conus medullaris and distal nerve roots in relation to the sacrum. The conus contains the sacral and coccygeal segments of the spinal cord (Taylor and Coolican, 1988). Criteria for recognizing the conus on computed tomography (CT) scans have been published by Grogan et al. (1984). The radiological properties of the structure have been studied through magnetic resonance imaging (MRI) (Saifuddin et al., 1997). -

227 INTRODUCTION: Diastematomyelia (Also Known As a Split Cord Malformation) Is a Rare Dysraphic Lesion of the Spinal Cord in W
Role of rehabilitation a case of diastematomyelia Stanescu Ioana¹, Kallo Rita¹, Bulboaca Adriana³, Dogaru Gabriela ² 1.Rehabilitation Hospital Cluj, Neurology Department 2.Rehabilitation Hospital Cluj, Physical Medicine and Rehabilitation Department 3.University of Medecine and Pharmacy Cluj - Physiopathology Department Balneo Research Journal DOI: http://dx.doi.org/10.12680/balneo.2017.156 Vol.8, No.4, December 2017 p: 227 – 230 Corresponding author: Gabriela Dogaru, E-mail address: [email protected] Abstract Diastematomyelia (split cord malformation) is a rare dysraphic lesion in which a part of the spinal cord is split in the sagittal plane into two hemicords, a bony, cartilagenous or fibrous spur projecting through the dura mater is visible in 33% of cases. Vertebral anomalies (spina bifida) are common. It occurs usually between D9 and S1. Classificatin includes two types: type 1 with a duplicated dural sac, with common midline spur, usually symptomatic, and type 2 with a single dural sac and usually less symptomatic. The majority of patients are presenting with tethered cord syndrome (neurologic deficits in the lower limbs and perineum). MRI is the modality of choice for diagnosis. In symptomatic cases, surgical release of the cord and resection of spur with repair of dura are performed, with good results. We present a case of a pauci-symptomatic type 1 dyastematomyelia, manifested by intermittent and resistant lumbar pain, in which physiotherapy during rehabilitation program have shown to improve pain intensity. Key words: spinal cord malformation, dyastematomyelia, lumbar spine, INTRODUCTION: Diastematomyelia (also Intramedullary tumours associated with known as a split cord malformation) is a rare diastematomyelia have been rarely described and dysraphic lesion of the spinal cord in which a part associated conditions like a tethered cord, inclusion of the spinal cord is split in the sagittal plane into dermoid, lipoma, syringo-hydromyelia and Chiari two hemicords. -
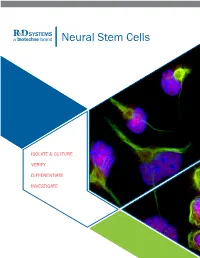
Neural Stem Cells
RnDSy-lu-2945 Neural Stem Cells ISOLATE & CULTURE VERIFY DIFFERENTIATE INVESTIGATE ISOLATE AND CULTURE Neural Stem cells (NSCs) require specialized media and growth factors to ensure efficient expansion. In addition to multipotent mouse and rat primary cortical stem cells, Bio-Techne offers a variety of serum-free neural media supplements, growth factors, and small molecules to maintain and expand NSCs. N21-MAX Cell Culture Supplements and Substrates • Improved Cell Health: high-quality culture reagents to ensure better growth and differentiation • Efficient Growth: optimized to enhance neural cell growth in culture Product Catalog # N-2 Plus Media Supplement AR003 N-2 MAX Media Supplement AR009 N21-MAX Media Supplement AR008 N21-MAX Insulin Free Media Supplement AR010 Competitor N21-MAX Vitamin A Free Media Supplement AR012 Holo-Transferrin 2914-HT Human Fibronectin, CF 1918-FN Bovine Fibronectin, CF 1030-FN Recombinant Human Fibronectin Full, CF 4305-FN Recombinant Human Fibronectin Fragment 2 3225-FN Recombinant Human Fibronectin Fragment 3 3938-FN Recombinant Human Fibronectin Fragment 4 3624-FN Recombinant Human Fibronectin, GMP 4305-GMP Recombinant Human Fibronectin, ACFP ACFP4305 Increased Synaptic Puncta and Neurite Outgrowth of ® Cultrex Poly-L-Lysine 3438-100-01 Primary Neurons Cultured in N21-MAX. E18 rat hippo- campal neurons were grown for 21 days in vitro in media supplemented with either N21-MAX Media Supplement (Catalog # AR008) or the neural media supplement from the most widely-used competitor. Staining for Synaptotagmin (yellow) showed more robust synaptic puncta and increased neurite outgrowth in neurons cultured in N21-MAX compared to those cultured in competitor media. Cells were stained with a Mouse Anti-Rat Synaptotagmin-1 Monoclonal Antibody (Catalog # MAB4364) followed by the NorthernLights™ (NL)557-conjugated Donkey Anti-Mouse IgG Secondary Antibody (Catalog # NL007). -
Functional Anatomy of the Spinal Cord
European Course in Neuroradiology Module 1 - Anatomy and Embryology 16th Cycle Module 1 Functional Anatomy of the Spinal Cord No disclosures Johan Van Goethem Spinal Cord Spinal nerves • vertebral canal • conus medullaris: adult Th12- L1 • ventral and dorsal roots • filum terminale • cauda equina • intervertebral foramen • gray matter: anterior and posterior horns • each pair (31) innervates a • white matter: anterior, lateral and posterior tracts body segment: dermatomes • cervical (8 p.), thoracic (12 p.), lumbar (5 p.), sacral (5 p.) and coccygeal (1 p.) Dorsal Root Ganglion Dorsal Root Ganglion Elliot S. Krames et al 2014 Tiantian Guo et al 2019 Spinal cord anatomy Spinal cord anatomy Source: Prometheus • 3 ascending pathways Anatomical atlas • 2 descending pathways Source: Wikipedia 40-year-old woman So what is this? • gradual and uniform onset of • Lichtheim's disease • diminished pressure, vibration and touch sense • Lou Gehrig's disease • tingling and numbness of legs, arms and trunk that progressively worsens • Lyme disease • pain and temperature sense are normal • Devic’s disease • motor function is preserved, but slight ataxia And the answer is ... Let’s go back ... • gradual and uniform onset of • Lichtheim's disease • diminished pressure, vibration and touch sense • Lou Gehrig's disease • tingling and numbness of legs, arms and trunk that progressively worsens • Lyme disease • pain and temperature sense are normal • Devic’s disease • motor function is preserved, but slight ataxia Spinothalamic tract Spinothalamic tract • 3 -

Definitions of Traumatic Conus Medullaris and Cauda
Spinal Cord (2017) 55, 886–890 & 2017 International Spinal Cord Society All rights reserved 1362-4393/17 www.nature.com/sc ORIGINAL ARTICLE Definitions of traumatic conus medullaris and cauda equina syndrome: a systematic literature review E Brouwers1, H van de Meent2, A Curt3, B Starremans4, A Hosman5 and R Bartels1 Study design: A systematic review. Objectives: Conus medullaris syndrome (CMS) and cauda equina syndrome (CES) are well-known neurological entities. It is assumed that these syndromes are different regarding neurological and functional prognosis. However, literature concerning spinal trauma is ambiguous about the exact definition of the syndromes. Methods: A MEDLINE, EMBASE and Cochrane literature search was performed. We included original articles in which clinical descriptions of CMS and/or CES were mentioned in patients following trauma to the thoracolumbar spine. Results: Out of the 1046 articles, we identified 14 original articles concerning patients with a traumatic CMS and/or CES. Based on this review and anatomical data from cadaveric and radiological studies, CMS and CES could be more precisely defined. Conclusion: CMS may result from injury of vertebrae Th12–L2, and it involves damage to neural structures from spinal cord segment Th12 to nerve root S5. CES may result from an injury of vertebrae L3–L5, and it involves damage to nerve roots L3–S5. This differentiation between CMS and CES is necessary to examine the hypothesis that CES patients tend to have a better functional outcome. Spinal Cord (2017) 55, 886–890; doi:10.1038/sc.2017.54; published online 23 May 2017 INTRODUCTION described clinical symptoms that were caused by compression of the Traumatic injuries of the thoracolumbar spine can result in conus CE and named this as ‘cauda equina compression syndrome’.20 From medullaris syndrome (CMS) or cauda equina syndrome (CES).