Application of High Performance Liquid Chromatography (HPLC) and Fourier Transform Infrared (FTIR) Spectroscopy to Swiss-Typ
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Using Visible and Near-Infrared Reflectance Spectroscopy and Differential Scanning Calorimetry to Study Starch, Protein, and Temperature Effects on Bread Staling
Using Visible and Near-Infrared Reflectance Spectroscopy and Differential Scanning Calorimetry to Study Starch, Protein, and Temperature Effects on Bread Staling Feng Xie,1 Floyd E. Dowell,2,3 and Xiuzhi S. Sun1 ABSTRACT Cereal Chem. 81(2):249–254 Starch, protein, and temperature effects on bread staling were inves- retrograded amylose-lipid complex was limited. Two important wave- tigated using visible and near-infrared spectroscopy (NIRS) and differ- lengths, 550 nm and 1,465 nm, were key for NIRS to successfully classify ential scanning calorimetry (DSC). Bread staling was mainly due to the starch-starch (SS) and starch-protein (SP) bread based on different amylopectin retrogradation. NIRS measured amylopectin retrogradation colors and protein contents in SS and SP. Low temperature dramatically accurately in different batches. Three important wavelengths, 970 nm, accelerated the amylopectin retrogradation process. Protein retarded bread 1,155 nm, and 1,395 nm, were associated with amylopectin retrogra- staling, but not as much as temperature. The starch and protein interaction dation. NIRS followed moisture and starch structure changes when was less important than the starch retrogradation. Protein hindered the amylopectin retrograded. The amylose-lipid complex changed little from bread staling process mainly by diluting starch and retarding starch retro- one day after baking. The capability of NIRS to measure changes in the gradation. Bread staling is a complex process that occurs during bread and starch (Osborne and Douglas 1981; Davies and Miller 1988; storage. It is a progressive deterioration of qualities such as taste, Millar et al 1996). Therefore, NIRS has the potential to provide fun- firmness, etc. -

Comparison Between FTIR and Boehm Titration for Activated Carbon Functional Group Quantification
Comparison Between FTIR and Boehm Titration for Activated Carbon Functional Group Quantification Chad Spreadbury, Regina Rodriguez, and David Mazyck College of Engineering, University of Florida Activated carbon (AC) is a proven effective adsorbent of contaminants in the air and water phases. This effectiveness is due to the large surface area of AC which hosts functional groups. Particularly, oxygen functional groups (e.g. carboxyls, lactones, phenols, carbonyls) have been noted as important in mercury removal. Hence, determining the quantities of these groups is crucial when AC is used for this purpose. Currently, Boehm titration is the most common method for determining the number of these functional groups. However, this test method is susceptible to high user error and require a lengthy procedure and run time. On the other hand, Fourier transform infrared (FTIR) spectroscopy excels at analyzing the functionality of an AC surface. This study investigates whether or not a correlation between Boehm titration and FTIR can be determined using quantitative and qualitative assessment. The results indicate that while using the current methodology for Boehm titrations and FTIR analysis, there is no clear correlation. However, this study does not rule out that a correlation may indeed exist between these test methods that can be elucidated with improved methodology that accounts for the unique physical and chemical characteristics of carbon. INTRODUCTION mercury (Hg0) to create oxidized mercury (Hg2+), which then undergoes chemisorption with the AC surface. ctivated carbon is used globally as an effective Boehm titrations and Fourier transform infrared (FTIR) adsorbent for removing contaminants like mercury spectroscopy are two common testing procedures for A(Hg) from air and water phases. -
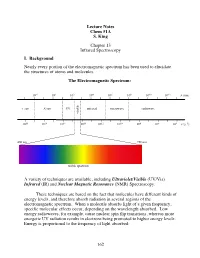
162 Lecture Notes Chem 51A S. King Chapter 13 Infrared Spectroscopy I
Lecture Notes Chem 51A S. King Chapter 13 Infrared Spectroscopy I. Background Nearly every portion of the electromagnetic spectrum has been used to elucidate the structures of atoms and molecules. The Electromagnetic Spectrum: 10!2 100 102 104 106 108 1010 1012 $ (nm) "!ray X-ray UV infrared microwave radiowave visible 1020 1018 1016 1014 1012 1010 108 106 104 # (s!1) 400 nm 750 nm ! visible spectrum A variety of techniques are available, including Ultraviolet/Visible (UV/Vis) Infrared (IR) and Nuclear Magnetic Resonance (NMR) Spectroscopy. These techniques are based on the fact that molecules have different kinds of energy levels, and therefore absorb radiation in several regions of the electromagnetic spectrum. When a molecule absorbs light of a given frequency, specific molecular effects occur, depending on the wavelength absorbed. Low energy radiowaves, for example, cause nuclear spin flip transitions, whereas more energetic UV radiation results in electrons being promoted to higher energy levels. Energy is proportional to the frequency of light absorbed: 162 Molecular effects associated with different regions of the EM spectrum: Wavelength (!) Energy/mole Molecular effects 10"10 meter gamma rays 106 kcal 10"8 meter X-rays 104 kcal ionization vaccum UV 102 kcal near UV electronic transitions 10"6 meter visible 10 kcal infrared 1 kcal molecular vibrations 10"4 meter (IR) 10"2 kcal 10"2 meter microwave rotational motion 10"4 kcal 0 10 meter radio 10"6 kcal nuclear spin transitions 2 10 meter II. IR Spectroscopy IR radiation causes groups of atoms to vibrate with respect to the bonds that connect them. -
Infrared and UV-Visible Time-Resolved Techniques for the Study of Tetrapyrrole-Based Proteins
Infrared and UV-visible time-resolved techniques for the study of tetrapyrrole-based proteins A thesis submitted to The University of Manchester for the degree of Doctor of Philosophy (PhD) in the Faculty of Life Sciences 2013 Henry John Russell Contents List of Tables .......................................................................................................................................................................... 5 List of Figures ........................................................................................................................................................................ 6 Abstract ...................................................................................................................................................................................10 Declaration ............................................................................................................................................................................11 Copyright Statement ........................................................................................................................................................11 Acknowledgements ..........................................................................................................................................................12 Abbreviations and Symbols ..........................................................................................................................................13 Preface and Thesis Structure .......................................................................................................................................16 -

And Raman Spectroscopy IR and Raman Spectroscopy Measure the Energy Difference of Vibrational Energy Levels in Molecules, They Are Energy Sensitive Methods
Infrared (IR) and Raman Spectroscopy IR and Raman spectroscopy measure the energy difference of vibrational energy levels in molecules, they are energy sensitive methods. They are based on interactions of electromagnetic radiation and material but the main differences between these two spectroscopic techniques are the physical effects. For infrared spectroscopy the absorption process of polychromatic IR radiation due to the molecule cause a change in the dipole moment of the molecule and the molecule will be translated from a ground state to a vibrational excited state. Whereas the monochromatic radiation, for Raman spectroscopy, is inelastic scattered at the electron shell of the molecule which changes the molecular polarizability and the radiation will be shifted in frequency (light source: UV, VIS or NIR lasers). The “Raman effect” was discovered by the Indian physicist, C. V. Raman inn 1928. Selection rules: Whether or not a vibrational mode is active or inactive for one of these complementary (inversely related) techniques depends on the symmetry of the molecules. For example, homonuclear diatomic molecules are not IR active, because they have no dipole moment, but they are Raman active. Because of the stretching and contraction of the bond changes the interactions between the electrons and nuclei, this causes a change of polarizability. For highly symmetric polyatomic molecules possessing a center of inversion, the bands are IR active (Raman inactive) for asymmetric vibrations to i and for symmetric vibrations to i the bands are Raman active (IR inactive). A mode can be IR active, Raman inactive and vice-versa however not at the same time. This fact is named as mutual exclusion rule. -

Fourier Transform Infrared Spectroscopy
FOURIER TRANSFORM INFRARED SPECTROSCOPY Purpose and Scope: This document describes the procedures and policies for using the MSE Bruker FTIR Spectrometer. The scope of this document is to establish user procedures. Instrument maintenance and repair are outside the scope of this document. Responsibilities: This document is maintained by the department Lab manager. The Lab Manager is responsible for general maintenance and for arranging repair when necessary. If you feel that the instrument is in need of repair or is not operating correctly, please notify the Lab Manager immediately. The Lab Manger will operate the instruments according to the procedures set down in this document and will provide instruction and training to users within the department. Users are responsible for using the instrument described according to these procedures. These procedures assume that the user has had at least one training session. Warnings and precautions: • When using the EGA-TGA Cell remember that it is hot. The cell and the line from the TGA to the cell are operated at 200C. • When using the EGA-TGA cell please remember to turn off the heater by pressing the down arrow on the controller until the set point reads “off”. You will be shown how to do this during training. • User are NOT cleared to change out fixtures. Only lab personnel are allowed to install or remove the ATR, the pellet holder, the EGA-TGA Cell or any other fixtures. We recommend that you notify lab personnel the day before your scheduled measurement to ensure it is ready. • For measuring KBr pellets: Users are expected to make/have their own pellet equipment. -

What-Is -Ft-Ir.Pdf
What is FT‐IR? Infrared (IR) spectroscopy is a chemical analytical technique, which measures the infrared intensity versus wavelength (wavenumber) of light. Based upon the wavenumber, infrared light can be categorized as far infrared (4 ~ 400cm‐1), mid infrared (400 ~ 4,000cm‐1) and near infrared (4,000 ~ 14,000cm‐1). Infrared spectroscopy detects the vibration characteristics of chemical functional groups in a sample. When an infrared light interacts with the matter, chemical bonds will stretch, contract and bend. As a result, a chemical functional group tends to adsorb infrared radiation in a specific wavenumber range regardless of the structure of the rest of the molecule. For example, the C=O stretch of a carbonyl group appears at around 1700cm‐1 in a variety of molecules. Hence, the correlation of the band wavenumber position with the chemical structure is used to identify a functional group in a sample. The wavenember positions where functional groups adsorb are consistent, despite the effect of temperature, pressure, sampling, or change in the molecule structure in other parts of the molecules. Thus the presence of specific functional groups can be monitored by these types of infrared bands, which are called group wavenumbers. The early‐stage IR instrument is of the dispersive type, which uses a prism or a grating monochromator. The dispersive instrument is characteristic of a slow scanning. A Fourier Transform Infrared (FTIR) spectrometer obtains infrared spectra by first collecting an interferogram of a sample signal with an interferometer, which measures all of infrared frequencies simultaneously. An FTIR spectrometer acquires and digitizes the interferogram, performs the FT function, and outputs the spectrum. -

Mid-Infrared Spectroscopy As a Valuable Tool to Tackle Food Analysis: a Literature Review on Coffee, Dairies, Honey, Olive Oil and Wine
foods Review Mid-Infrared Spectroscopy as a Valuable Tool to Tackle Food Analysis: A Literature Review on Coffee, Dairies, Honey, Olive Oil and Wine Eduarda Mendes and Noélia Duarte * Research Institute for Medicines (iMED.Ulisboa), Faculty of Pharmacy, Universidade de Lisboa, Av. Prof. Gama Pinto, 1649-003 Lisbon, Portugal; [email protected] * Correspondence: [email protected] Abstract: Nowadays, food adulteration and authentication are topics of utmost importance for con- sumers, food producers, business operators and regulatory agencies. Therefore, there is an increasing search for rapid, robust and accurate analytical techniques to determine the authenticity and to detect adulteration and misrepresentation. Mid-infrared spectroscopy (MIR), often associated with chemometric techniques, offers a fast and accurate method to detect and predict food adulteration based on the fingerprint characteristics of the food matrix. In the first part of this review the basic concepts of infrared spectroscopy, sampling techniques, as well as an overview of chemometric tools are summarized. In the second part, recent applications of MIR spectroscopy to the analysis of foods such as coffee, dairy products, honey, olive oil and wine are discussed, covering a timespan from 2010 to mid-2020. The literature gathered in this article clearly reveals that the MIR spectroscopy associated with attenuated total reflection acquisition mode and different chemometric tools have been broadly applied to address quality, authenticity and adulteration issues. This technique has the advantages of being simple, fast and easy to use, non-destructive, environmentally friendly and, in Citation: Mendes, E.; Duarte, N. Mid-Infrared Spectroscopy as a the future, it can be applied in routine analyses and official food control. -

Application of Spectroscopic UV-Vis and FT-IR
molecules Article Application of Spectroscopic UV-Vis and FT-IR Screening Techniques Coupled with Multivariate Statistical Analysis for Red Wine Authentication: Varietal and Vintage Year Discrimination Elisabeta-Irina Geană 1 , Corina Teodora Ciucure 1, Constantin Apetrei 2,* and Victoria Artem 3 1 National R&D Institute for Cryogenics and Isotopic Technologies—ICIT Rm. Valcea, 4th Uzinei Street, PO Raureni, Box 7, 240050 Rm. Valcea, Romania; [email protected] (E.-I.G.); [email protected] (C.T.C.) 2 Physics and Environment, Department of Chemistry, Faculty of Science and Environment, “Dunarea de Jos” University of Galati, 111 Domneasca Street, RO-800008 Galati, Romania 3 Research Station for Viticulture and Oenology Murfatlar, Calea Bucuresti str., no. 2, Murfatlar, 905100 Constanta, Romania; [email protected] * Correspondence: [email protected]; Tel.: +40-727-580-914 Received: 8 November 2019; Accepted: 15 November 2019; Published: 17 November 2019 Abstract: One of the most important issues in the wine sector and prevention of adulterations of wines are discrimination of grape varieties, geographical origin of wine, and year of vintage. In this experimental research study, UV-Vis and FT-IR spectroscopic screening analytical approaches together with chemometric pattern recognition techniques were applied and compared in addressing two wine authentication problems: discrimination of (i) varietal and (ii) year of vintage of red wines produced in the same oenological region. UV-Vis and FT-IR spectra of red wines were registered for all the samples and the principal features related to chemical composition of the samples were identified. Furthermore, for the discrimination and classification of red wines a multivariate data analysis was developed. -

Relationships in Gas Chromatography—Fourier Transform Infrared Spectroscopy—Comprehensive and Multilinear Analysis
separations Article Relationships in Gas Chromatography—Fourier Transform Infrared Spectroscopy—Comprehensive and Multilinear Analysis Junaida Shezmin Zavahir 1 , Jamieson S. P. Smith 1, Scott Blundell 2, Habtewold D. Waktola 1 , Yada Nolvachai 1 , Bayden R. Wood 3 and Philip J. Marriott 1,* 1 Australian Centre for Research on Separation Science, School of Chemistry, Monash University, Wellington Road, Clayton, Melbourne, VIC 3800, Australia; [email protected] (J.S.Z.); [email protected] (J.S.P.S.); [email protected] (H.D.W.); [email protected] (Y.N.) 2 Monash Analytical Platform, School of Chemistry, Monash University, Wellington Road, Clayton, Melbourne, VIC 3800, Australia; [email protected] 3 Centre for Biospectroscopy, School of Chemistry, Monash University, Wellington Road, Clayton, Melbourne, VIC 3800, Australia; [email protected] * Correspondence: [email protected]; Tel.: +61-3-9905-9630; Fax: +61-3-9905-8501 Received: 13 April 2020; Accepted: 8 May 2020; Published: 13 May 2020 Abstract: Molecular spectroscopic detection techniques, such as Fourier transform infrared spectroscopy (FTIR), provides additional specificity for isomers where often mass spectrometry (MS) fails, due to similar fragmentation patterns. A hyphenated system of gas chromatography (GC) with FTIR via a light-pipe interface is reported in this study to explore a number of GC–FTIR analytical capabilities. Various compound classes were analyzed—aromatics, essential oils and oximes. Variation in chromatographic peak parameters due to the light-pipe was observed via sequentially-located flame ionization detection data. Unique FTIR spectra were observed for separated mixtures of essential oil isomers having similar mass spectra. Presentation of GC FTIR allows a × ‘comprehensive’-style experiment to be developed. -

IR and Raman Spectroscopy
IR and Raman spectroscopy Peter Hildebrandt Content 1. Basics of vibrational spectroscopy 2. Special approaches 3. Time-resolved methods 1. Basics of vibrational spectroscopy 1.1. Molecular vibrations and normal modes 1.2. Normal mode analysis 1.3. Probing molecular vibrations 1.3.1. Fourier-transform infrared spectroscopy 1.3.2. Raman spectroscopy 1.4. Infrared intensities 1.5. Raman intensities 1.1. Molecular vibrations and normal modes IR and Raman spectroscopy - vibrational spectroscopy: probing well-defined vibrations of atoms within a molecule Siebert & Hildebrandt, 2007) Motions of the atoms in a molecule are not random! well-defined number of vibrational degrees of freedom 3N-6 and 3N-5 for non-linear and linear molecules, respectively What controls the molecular vibrations and how are they characterized? 1.1. Molecular vibrations and normal modes Definition of the molecular vibrations: Eigenwert problem normal modes for a non-linear N-atomic molecule: 3N-6 normal modes example: benzene in each normal mode: all atoms vibrate with the same frequency but different amplitudes -1 -1 1(A1g): 3062 cm 14(E1u): 1037 cm Thus: normal modes are characterised by frequencies (given in cm-1) and the extent by which individual atoms (or coordinates) are involved. 1.1. Molecular vibrations and normal modes Determination of normal modes – a problem of classical physics Approach: Point masses connected with springs Harmonic motion Siebert & Hildebrandt, 2007) Crucial parameters determining the normal modes: - Geometry of the molecule (spatial arrangement of the spheres) - strength of the springs (force constants) very sensitive fingerprint of the molecular structure 1.2. Normal mode analysis A. -

Infrared Imaging and Spectroscopy Beyond the Diffraction Limit
Infrared Imaging and Spectroscopy Beyond the Diffraction Limit Andrea Centrone Center for Nanoscale Science and Technology, National Institute of Standards and Technology, 100 Bureau Drive, Gaithersburg, Maryland 20899, United States E-mail: [email protected] Abstract Progress in nanotechnology is enabled by and dependent on the availabil- ity of measurement methods with spatial resolution commensurate with nanomaterials’ length scales. Chemical imaging techniques, such as scatter- ing scanning near-field optical microscopy (s- SNOM) and photothermal- induced resonance (PTIR), have provided scientists with means of extract- ing rich chemical and structural information with nanoscale resolution. This review presents some basics of infrared spectroscopy and microscopy, fol- lowed by detailed descriptions of s-SNOM and PTIR working principles. Nanoscale spectra are compared with far-field macroscale spectra, which are widely used for chemical identification. Selected examples illustrate either technical aspects of the measurements or applications in materials science. Central to this review is the ability to record nanoscale infrared spectra because, although chemical maps enable immediate visualization, the spectra provide information to interpret the images and characterize the sample. The growing breadth of nanomaterials and biological applications suggest rapid growth for this field. Keywords s-SNOM, PTIR, RE-AFMIR, nanoscale infrared spectroscopy, nanomaterials, chemical composition 1 1. Introduction Over the past two decades advances in top-down fabrication methods (1, 2) and in bottom-up nanomaterial synthesis (3–5) have enabled the preparation of a plethora of nanomaterials. Because nanomaterials typically provide novel properties or improved performance with respect to their macroscopic counterparts (6–10), they are of great interest in fields such as electronics (11), photovoltaics (12), biology (13), therapeutics (8, 14), and many more.