Fourier Transform Infrared Spectroscopy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ADVANCED SPECTROMETER Introduction
ADVANCED SPECTROMETER Introduction In principle, a spectrometer is the simplest of scientific very sensitive detection and precise measurement, a real instruments. Bend a beam of light with a prism or diffraction spectrometer is a bit more complicated. As shown in Figure grating. If the beam is composed of more than one color of 1, a spectrometer consists of three basic components; a light, a spectrum is formed, since the various colors are collimator, a diffracting element, and a telescope. refracted or diffracted to different angles. Carefully measure The light to be analyzed enters the collimator through a the angle to which each color of light is bent. The result is a narrow slit positioned at the focal point of the collimator spectral "fingerprint," which carries a wealth of information lens. The light leaving the collimator is therefore a thin, about the substance from which the light emanates. parallel beam, which ensures that all the light from the slit In most cases, substances must be hot if they are to emit strikes the diffracting element at the same angle of inci- light. But a spectrometer can also be used to investigate cold dence. This is necessary if a sharp image is to be formed. substances. Pass white light, which contains all the colors of The diffracting element bends the beam of light. If the beam the visible spectrum, through a cool gas. The result is an is composed of many different colors, each color is dif- absorption spectrum. All the colors of the visible spectrum fracted to a different angle. are seen, except for certain colors that are absorbed by the gas. -

Using Visible and Near-Infrared Reflectance Spectroscopy and Differential Scanning Calorimetry to Study Starch, Protein, and Temperature Effects on Bread Staling
Using Visible and Near-Infrared Reflectance Spectroscopy and Differential Scanning Calorimetry to Study Starch, Protein, and Temperature Effects on Bread Staling Feng Xie,1 Floyd E. Dowell,2,3 and Xiuzhi S. Sun1 ABSTRACT Cereal Chem. 81(2):249–254 Starch, protein, and temperature effects on bread staling were inves- retrograded amylose-lipid complex was limited. Two important wave- tigated using visible and near-infrared spectroscopy (NIRS) and differ- lengths, 550 nm and 1,465 nm, were key for NIRS to successfully classify ential scanning calorimetry (DSC). Bread staling was mainly due to the starch-starch (SS) and starch-protein (SP) bread based on different amylopectin retrogradation. NIRS measured amylopectin retrogradation colors and protein contents in SS and SP. Low temperature dramatically accurately in different batches. Three important wavelengths, 970 nm, accelerated the amylopectin retrogradation process. Protein retarded bread 1,155 nm, and 1,395 nm, were associated with amylopectin retrogra- staling, but not as much as temperature. The starch and protein interaction dation. NIRS followed moisture and starch structure changes when was less important than the starch retrogradation. Protein hindered the amylopectin retrograded. The amylose-lipid complex changed little from bread staling process mainly by diluting starch and retarding starch retro- one day after baking. The capability of NIRS to measure changes in the gradation. Bread staling is a complex process that occurs during bread and starch (Osborne and Douglas 1981; Davies and Miller 1988; storage. It is a progressive deterioration of qualities such as taste, Millar et al 1996). Therefore, NIRS has the potential to provide fun- firmness, etc. -

Spectrum 100 Series User's Guide
MOLECULAR SPECTROSCOPY SPECTRUM 100 SERIES User’s Guide 2 . Spectrum 100 Series User’s Guide Release History Part Number Release Publication Date L1050021 A October 2005 Any comments about the documentation for this product should be addressed to: User Assistance PerkinElmer Ltd Chalfont Road Seer Green Beaconsfield Bucks HP9 2FX United Kingdom Or emailed to: [email protected] Notices The information contained in this document is subject to change without notice. Except as specifically set forth in its terms and conditions of sale, PerkinElmer makes no warranty of any kind with regard to this document, including, but not limited to, the implied warranties of merchantability and fitness for a particular purpose. PerkinElmer shall not be liable for errors contained herein for incidental consequential damages in connection with furnishing, performance or use of this material. Copyright Information This document contains proprietary information that is protected by copyright. All rights are reserved. No part of this publication may be reproduced in any form whatsoever or translated into any language without the prior, written permission of PerkinElmer, Inc. Copyright © 2005 PerkinElmer, Inc. Trademarks Registered names, trademarks, etc. used in this document, even when not specifically marked as such, are protected by law. PerkinElmer is a registered trademark of PerkinElmer, Inc. Spectrum, Spectrum 100, and Spectrum 100N are trademarks of PerkinElmer, Inc. Spectrum 100 Series User’s Guide . 3 Contents Contents............................................................................................... -

Experiment 2 Radiation in the Visible Spectrum Emission Spectra Can Be
Experiment 2 Radiation in the Visible Spectrum Emission spectra can be a unique fingerprint of an atom or molecule. The photon energies and wavelengths are directly related to the allowed quantum energy states of the system. In the following experiments we will examine the radiation given off by sources radiating in the visible region. We will be using a spectrometer produced by Ocean Optics. Light enters the spectrometer via a fiber optic cable. Inside the spectrometer a diffraction grating diffracts the different frequencies onto a CCD. The CCD basically ”counts” the photons according to wavelength. The data is transferred via a usb port to a PC. The Ocean Optics software displays the spectrum as counts versus wavelength. We will examine the spectra given off by the following sources: incandescent light filament, hydrogen, helium, various light emitting diodes and a laser pointer. You will also be given an unknown gas discharge tube and will need to identify the gas via its spectral emissions Pre Lab 1) Obtain a spectrum for hydrogen, mercury, sodium and neon gas emissions. The spectrum must contain an accurate listing of major emission lines (in nm), not simply a color photo of the emission. 2) Make yourself familiar with the manual for the spectrometer and the software. These are available via the following links: http://www.oceanoptics.com/technical/hr4000.pdf http://www.oceanoptics.com/technical/SpectraSuite.pdf These documents are also available on Black Board. 3) How does the Ocean Optics spectrometer work? In your answer list its three main components and describe what each component does. -

Comparison Between FTIR and Boehm Titration for Activated Carbon Functional Group Quantification
Comparison Between FTIR and Boehm Titration for Activated Carbon Functional Group Quantification Chad Spreadbury, Regina Rodriguez, and David Mazyck College of Engineering, University of Florida Activated carbon (AC) is a proven effective adsorbent of contaminants in the air and water phases. This effectiveness is due to the large surface area of AC which hosts functional groups. Particularly, oxygen functional groups (e.g. carboxyls, lactones, phenols, carbonyls) have been noted as important in mercury removal. Hence, determining the quantities of these groups is crucial when AC is used for this purpose. Currently, Boehm titration is the most common method for determining the number of these functional groups. However, this test method is susceptible to high user error and require a lengthy procedure and run time. On the other hand, Fourier transform infrared (FTIR) spectroscopy excels at analyzing the functionality of an AC surface. This study investigates whether or not a correlation between Boehm titration and FTIR can be determined using quantitative and qualitative assessment. The results indicate that while using the current methodology for Boehm titrations and FTIR analysis, there is no clear correlation. However, this study does not rule out that a correlation may indeed exist between these test methods that can be elucidated with improved methodology that accounts for the unique physical and chemical characteristics of carbon. INTRODUCTION mercury (Hg0) to create oxidized mercury (Hg2+), which then undergoes chemisorption with the AC surface. ctivated carbon is used globally as an effective Boehm titrations and Fourier transform infrared (FTIR) adsorbent for removing contaminants like mercury spectroscopy are two common testing procedures for A(Hg) from air and water phases. -

Orbitrap Fusion Tribrid Mass Spectrometer
MASS SPECTROMETRY Product Specifications Thermo Scientific Orbitrap Fusion Tribrid Mass Spectrometer Unmatched analytical performance, revolutionary MS architecture The Thermo Scientific™ Orbitrap Fusion™ mass spectrometer combines the best of quadrupole, Orbitrap, and linear ion trap mass analysis in a revolutionary Thermo Scientific™ Tribrid™ architecture that delivers unprecedented depth of analysis. It enables life scientists working with even the most challenging samples—samples of low abundance, high complexity, or difficult-to-analyze chemical structure—to identify more compounds faster, quantify them more accurately, and elucidate molecular composition more thoroughly. • Tribrid architecture combines quadrupole, followed by ETD or EThCD for glycopeptide linear ion trap, and Orbitrap mass analyzers characterization or HCD followed by CID • Multiple fragmentation techniques—CID, for small-molecule structural analysis. HCD, and optional ETD and EThCD—are available at any stage of MSn, with The ultrahigh resolution of the Orbitrap mass subsequent mass analysis in either the ion analyzer increases certainty of analytical trap or Orbitrap mass analyzer results, enabling molecular-weight • Parallelization of MS and MSn acquisition determination for intact proteins and confident to maximize the amount of high-quality resolution of isobaric species. The unsurpassed data acquired scan rate and resolution of the system are • Next-generation ion sources and ion especially useful when dealing with complex optics increase system ease of operation and robustness and low-abundance samples in proteomics, • Innovative instrument control software metabolomics, glycomics, lipidomics, and makes setup easier, methods more similar applications. powerful, and operation more intuitive The intuitive user interface of the tune editor The Orbitrap Fusion Tribrid MS can perform and method editor makes instrument calibration a wide variety of analyses, from in-depth and method development easier. -

Mass Spectrometer
CLASSICAL CONCEPT REVIEW 6 Mass Spectrometer One of several devices currently used to measure the charge-to-mass ratio q m of charged atoms and molecules is the mass spectrometer. The mass spectrometer is used to find the charge-to-mass ratio of ions of known charge by measuring> the radius of their circular orbits in a uniform magnetic field. Equation 3-2 gives the radius of R for the circular orbit of a particle of mass m and charge q moving with speed u in a magnetic field B that is perpendicular to the velocity of the particle. Figure MS-1 shows a simple schematic drawing of a mass spectrometer. Ions from an ion source are accelerated by an electric field and enter a uniform magnetic field produced by an electromagnet. If the ions start from rest and move through a poten- tial DV, their kinetic energy when they enter the magnetic field equals their loss in potential energy, qDV: B out 1 mu2 = qDV MS-1 2 R The ions move in a semicircle of radius R given by Equation 3-2 and exit through a P P narrow aperture into an ion detector (or, in earlier times, a photographic plate) at point 1 u 2 P2, a distance 2R from the point where they enter the magnet. The speed u can be eliminated from Equations 3-2 and MS-1 to find q m in terms of DV, B, and R. The + + q result is Source > – + q 2DV ∆V = 2 2 MS-2 m B R MS-1 Schematic drawing of a mass spectrometer. -

Application of High Performance Liquid Chromatography (HPLC) and Fourier Transform Infrared (FTIR) Spectroscopy to Swiss-Typ
Application of High Performance Liquid Chromatography (HPLC) and Fourier Transform Infrared (FTIR) Spectroscopy to Swiss-type Cheese Split Defects THESIS Presented in Partial Fulfillment of the Requirements for the Master of Science Degree in the Graduate School of The Ohio State University By Feifei Hu Graduate Program in Food Science and Technology The Ohio State University 2012 Master's Examination Committee: Dr. W. James Harper, Advisor Dr. Luis E. Rodriguez-Saona Dr. Michael E. Mangino Copyright Feifei Hu 2012 Abstract Splits, slits and cracks are a continuing problem in the US Swiss Cheese industry and cause downgrading of the cheese, bringing economic loss. The defects generally occur during secondary fermentation in cold storage, which is known to be an issue caused by many factors. Many chemical and physical changes can be indicators to help predicting the defects. Various techniques have been applied to test different compounds in cheese. HPLC is one of the reference methods which have been applied in cheese chemistry for the past decades. Its application involves in the qualification and quantification of organic acids, amino acids and peptides, and sugars in dairy products. However, limited research has been conducted on a rapid and easy testing regimen to investigate split defects in Swiss-type cheese. For over fifty years, researchers have investigated the application of Fourier Transform Infrared (FTIR) Spectroscopy in various food samples, including cheeses and other dairy products because FTIR spectroscopy gives fast, simple and comprehensive detection of chemical compounds. There is great potential for applying FTIR spectroscopy in developing a rapid test method to detect and predict the occurrence of split defects. -

Development and Applications of a Real-Time Magnetic Electron
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 12-13-2017 Development and Applications of a Real-time Magnetic Electron Energy Spectrometer for Use with Medical Linear Accelerators Paul Ethan Maggi Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Health and Medical Physics Commons Recommended Citation Maggi, Paul Ethan, "Development and Applications of a Real-time Magnetic Electron Energy Spectrometer for Use with Medical Linear Accelerators" (2017). LSU Doctoral Dissertations. 4180. https://digitalcommons.lsu.edu/gradschool_dissertations/4180 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. DEVELOPMENT AND APPLICATIONS OF A REAL-TIME MAGNETIC ELECTRON ENERGY SPECTROMETER FOR USE WITH MEDICAL LINEAR ACCELERATORS A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor in Philosophy in The Department of Physics and Astronomy by Paul Ethan Maggi B.S., California Polytechnic State University, San Luis Obispo, 2012 May 2018 ACKNOWLEDGEMENTS I thank Kenneth Hogstrom for providing the initial idea for this project, as well as partial funding support. I thank Edison Liang of Rice University for providing the magnet block and measurements of the magnetic field. I thank my adviser, Kip Matthews for his advice, guidance, and patience during my tenure at LSU. -
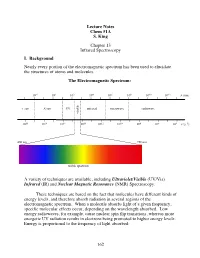
162 Lecture Notes Chem 51A S. King Chapter 13 Infrared Spectroscopy I
Lecture Notes Chem 51A S. King Chapter 13 Infrared Spectroscopy I. Background Nearly every portion of the electromagnetic spectrum has been used to elucidate the structures of atoms and molecules. The Electromagnetic Spectrum: 10!2 100 102 104 106 108 1010 1012 $ (nm) "!ray X-ray UV infrared microwave radiowave visible 1020 1018 1016 1014 1012 1010 108 106 104 # (s!1) 400 nm 750 nm ! visible spectrum A variety of techniques are available, including Ultraviolet/Visible (UV/Vis) Infrared (IR) and Nuclear Magnetic Resonance (NMR) Spectroscopy. These techniques are based on the fact that molecules have different kinds of energy levels, and therefore absorb radiation in several regions of the electromagnetic spectrum. When a molecule absorbs light of a given frequency, specific molecular effects occur, depending on the wavelength absorbed. Low energy radiowaves, for example, cause nuclear spin flip transitions, whereas more energetic UV radiation results in electrons being promoted to higher energy levels. Energy is proportional to the frequency of light absorbed: 162 Molecular effects associated with different regions of the EM spectrum: Wavelength (!) Energy/mole Molecular effects 10"10 meter gamma rays 106 kcal 10"8 meter X-rays 104 kcal ionization vaccum UV 102 kcal near UV electronic transitions 10"6 meter visible 10 kcal infrared 1 kcal molecular vibrations 10"4 meter (IR) 10"2 kcal 10"2 meter microwave rotational motion 10"4 kcal 0 10 meter radio 10"6 kcal nuclear spin transitions 2 10 meter II. IR Spectroscopy IR radiation causes groups of atoms to vibrate with respect to the bonds that connect them. -

Vernier Spectrometer Solution, Proceed Directly to the Calibrate Section Below
Select the Type of Data (or Units) You Want to Measure The default data type is absorbance. If you want to measure the absorbance of a Vernier Spectrometer solution, proceed directly to the Calibrate section below. (Order Code: V-SPEC) If you want to measure %T or intensity, do the following: 1. Choose Change Units ► Spectrometer from the Experiment menu. 2. Select the unit or data type you wish to measure. The Vernier Spectrometer is a portable visible light spectrometer. Calibrate (Not Required if Measuring Intensity) 1. To calibrate the Spectrometer, choose Calibrate ► Spectrometer from the What is Included with the Vernier Spectrometer? Experiment menu. Note: For best results, allow the Spectrometer to warm up for a Spectrometer (with light source/cuvette holder attached) minimum of five minutes. One package of 15 plastic cuvettes and lids 2. Fill a cuvette about ¾ full with distilled water (or the solvent being used in the USB cable experiment) to serve as the blank. After the Spectrometer has warmed up, place the blank cuvette in the Spectrometer. Align the cuvette so the clear side of the Software Requirements cuvette is facing the light source. Logger Pro® 3 (version 3.8.5 or newer) software is required if you are using a 3. Follow the instructions in the dialog box to complete the calibration, and then computer. LabQuest App version 2.0, or newer, is required if you are using click . LabQuest® 2. LabQuest App 1.1, or newer, is required if you are using the original LabQuest. Visit the downloads section of www.vernier.com to update your software. -

Real-Time Magnetic Electron Energy Spectrometer for Use with Medical Linear Accelerators P
ISBN 978-3-95450-180-9 Proceedings of NAPAC2016, Chicago, IL, USA TUPOA38 REAL-TIME MAGNETIC ELECTRON ENERGY SPECTROMETER FOR USE WITH MEDICAL LINEAR ACCELERATORS P. E. Maggi†, K. R. Hogstrom, K. L. Matthews II, Louisiana State University, Baton Rouge, LA 70803, USA R. L. Carver, Mary Bird Perkins Cancer Center, Baton Rouge, LA 70809, USA Abstract that there is a low-energy tail present in the spectrum [1] Accelerator characterization and quality assurance is an as a result of beam conditioning for therapeutic use. Addi- integral part of electron linear accelerator (linac) use in a tionally, measurements by Kok et al [4] have shown that medical setting. The current clinical method for radiation spectra for certain traveling-wave linacs (Elekta, Phillips) metrology of electron beams (dose on central axis versus can have further spectral deviations violating the Gaussi- depth in water) only provides a surrogate for the underly- an assumption; this is due to accelerator tuning parame- ing performance of the accelerator and does not provide ters (e.g. High Powered Phase Shifter) relating to the RF direct information about the electron energy spectrum. recycling system. A magnetic spectrometer has the poten- We have developed an easy to use real-time magnetic tial to simplify beam measurements, reduce the time electron energy spectrometer for characterizing the elec- needed for beam matching, and provides more infor- tron beams of medical linacs. Our spectrometer uses a mation about the electron beam. 0.57 T permanent magnet block as the dispersive element and scintillating fibers coupled to a CCD camera as the SPECTROMETER HARDWARE position sensitive detector.