Growth Rate Variability and Lipofuscin Accumulation Rates in the Blue Crab Callinectes Sapidus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blue Crab (Callinectes Sapidus) Use of the Ship/Trinity/Tiger Shoal
ACCESS NUMBER: 32806-35161 STUDY TITLE: Ship Shoal: Sand, Shrimp, and Seatrout REPORT TITLE: Blue Crab (Callinectes sapidus) Use of the Ship/Trinity/Tiger Shoal Complex as a Nationally Important Spawning/Hatching/Foraging Ground: Discovery, Evaluation, and Sand Mining Recommendations based on Blue Crab, Shrimp, and Spotted Seatrout Findings CONTRACT NUMBER: 1435-01-04-CA-32806-35161 (M05AZ10660) SPONSORING OCS REGION: Gulf of Mexico APPLICABLE PLANNING AREA: Central FISCAL YEARS OF PROJECT FUNDING: 2004-2009 COMPLETION DATE OF REPORT: December 2009 COSTS: FY 2004-2009, $145,778; CUMMULATIVE PROJECT COSTS: $145,778 PROJECT MANAGER: R. E. Condrey AFFILIATION: Louisiana State University, Department of Oceanography and Coastal Sciences, School of the Coast and Environment ADDRESS: Energy, Coast, and Environment Building, Louisiana State University, Baton Rouge, LA 70803 PRINCIPAL INVESTIGATOR: R.E. Condrey KEY WORDS: Gulf of Mexico, Ship Shoal, Trinity Shoal, Tiger Shoal, Louisiana, sand mining, blue crab, white shrimp, brown shrimp, spotted seatrout, condition factor, fecundity, spawning grounds, coastal restoration BACKGROUND: The Minerals Management Service (MMS) is addressing the recent demand for U.S. continental shelf sand resources for coastal erosion management, a critical challenge to Louisiana’s ecosystems and economies. Louisiana considers barrier island restoration as a promising way to combat wetland loss, with sand mined from Ship Shoal as the most feasible sediment source. Additional sand resources on Trinity and Tiger Shoals are also being considered. Since this area supports major demersal fisheries on white and brown shrimp (Litopenaeus setiferus and Farfantepenaeus aztecus), the present study was conducted as part of a larger effort to understand the sand-mining ecology of the Ship/Trinity/Tiger Shoal Complex (STTSC). -

Dimorphism and the Functional Basis of Claw Strength in Six Brachyuran Crabs
J. Zool., Lond. (2001) 255, 105±119 # 2001 The Zoological Society of London Printed in the United Kingdom Dimorphism and the functional basis of claw strength in six brachyuran crabs Steve C. Schenk1 and Peter C. Wainwright2 1 Department of Biological Science, Florida State University, Tallahassee, Florida 32306, U.S.A. 2 Section of Evolution and Ecology, University of California, Davis, CA 95616, U.S.A. (Accepted 7 November 2000) Abstract By examining the morphological basis of force generation in the chelae (claws) of both molluscivorous and non-molluscivorous crabs, it is possible to understand better the difference between general crab claw design and the morphology associated with durophagy. This comparative study investigates the mor- phology underlying claw force production and intraspeci®c claw dimorphism in six brachyuran crabs: Callinectes sapidus (Portunidae), Libinia emarginata (Majidae), Ocypode quadrata (Ocypodidae), Menippe mercenaria (Xanthidae), Panopeus herbstii (Xanthidae), and P. obesus (Xanthidae). The crushers of the three molluscivorous xanthids consistently proved to be morphologically `strong,' having largest mechan- ical advantages (MAs), mean angles of pinnation (MAPs), and physiological cross-sectional areas (PCSAs). However, some patterns of variation (e.g. low MA in C. sapidus, indistinguishable force generation potential in the xanthids) suggested that a quantitative assessment of occlusion and dentition is needed to understand fully the relationship between force generation and diet. Interspeci®c differences in force generation potential seemed mainly to be a function of differences in chela closer muscle cross- sectional area, due to a sixfold variation in apodeme area. Intraspeci®c dimorphism was generally de®ned by tall crushers with long in-levers, though O. -

Chinese Mitten Crab (Eriocheir Sinensis)
www.nonnativespecies.org For definitive identification, contact: [email protected] Chinese mitten crab (Eriocheir sinensis) Synonyms: - big sluiceway crab, Chinese freshwater edible crab, Chinese mittenhanded crab, Chinese mit- ten-handed crab, Chinese river crab, crabe chi- nois, mitten crab, river crab, Shanghai crab, villus crab - Eriocheir japonicas, E. leptognathus, E. rectus Consignments likely to come from: unknown Use: may be used for human consumption Identification difficulty: easy Identification information: - the only freshwater crab present in Britain - body (carapace) olive-green-brown, up to 8cm wide - pincers covered in a mat of fine hair resembling mittens - legs long and hairy Key ID Features Pincers covered in a mat Body (carapace) olive- of fine hair, giving the green-brown, up to 8 cm appearance of mittens wide Up to 8 cm Legs long and hairy Male and female Chinese mitten crabs are similar in appearance except their undersides Underside Underside of female of male Similar species Three other crab species are commonly imported to Britain but these are unlikely to be confused with Chinese mitten crab. Eriocheir sinensis Metacarcinus magister (Chinese mitten crab) (Dungeness crab) for comparison Body up tp to 8cm Legs long across and hairy Body up tp to 25cm across, usually under 20cm Body beige to light brown in Body (carapace) olive- Pincers covered in a mat colour with blue edges green-brown of fine hair, giving the appearance of mittens Callinectes sapidus Portunus pelagicus (Atlantic blue crab) (blue swimmer crab) Legs blue, body greyish / Body up tp to 27cm Vary in colour from dark Body up tp to 21cm greenish brown across brown, blue and purple across Pair of long, pointed Paddle shaped rear legs spines at lateral edges Paddle shaped rear legs of body Photos from: Almandine, Children’s Museum of Indianapolis, Dan Boone—United States Fish and Wildlife Service (all via Wikimedia Commons), & APHA . -

A New Pathogenic Virus in the Caribbean Spiny Lobster Panulirus Argus from the Florida Keys
DISEASES OF AQUATIC ORGANISMS Vol. 59: 109–118, 2004 Published May 5 Dis Aquat Org A new pathogenic virus in the Caribbean spiny lobster Panulirus argus from the Florida Keys Jeffrey D. Shields1,*, Donald C. Behringer Jr2 1Virginia Institute of Marine Science, The College of William & Mary, Gloucester Point, Virginia 23062, USA 2Department of Biological Sciences, Old Dominion University, Norfolk, Virginia 23529, USA ABSTRACT: A pathogenic virus was diagnosed from juvenile Caribbean spiny lobsters Panulirus argus from the Florida Keys. Moribund lobsters had characteristically milky hemolymph that did not clot. Altered hyalinocytes and semigranulocytes, but not granulocytes, were observed with light microscopy. Infected hemocytes had emarginated, condensed chromatin, hypertrophied nuclei and faint eosinophilic Cowdry-type-A inclusions. In some cases, infected cells were observed in soft con- nective tissues. With electron microscopy, unenveloped, nonoccluded, icosahedral virions (182 ± 9 nm SD) were diffusely spread around the inner periphery of the nuclear envelope. Virions also occurred in loose aggregates in the cytoplasm or were free in the hemolymph. Assembly of the nucleocapsid occurred entirely within the nucleus of the infected cells. Within the virogenic stroma, blunt rod-like structures or whorls of electron-dense granular material were apparently associated with viral assembly. The prevalence of overt infections, defined as lethargic animals with milky hemolymph, ranged from 6 to 8% with certain foci reaching prevalences of 37%. The disease was transmissible to uninfected lobsters using inoculations of raw hemolymph from infected animals. Inoculated animals became moribund 5 to 7 d before dying and they began dying after 30 to 80 d post-exposure. -

Blue Crab. U.S
~C' k""~-- - -- - S..- - _ __ - -. .54.5 .tS-'--------- - - - -tt Coastal Ecology Group Fish and Wildlife Service . Waterways Experiment Station U.S. Department of the Interior U.S. Army Corps of Engineers c-ýz N7cw jo r ýs Biological Report 82(11.100) TR EL-82-4 March 1989 Species Profiles: Life Histories and Environmental Requirements of Coastal Fishes and Invertebrates (Mid-Atlantic) BLUE CRAB by Jennifer Hill, Dean L. Fowler, and Michael J. Van Den Avyle Georgia Cooperative Fish and Wildlife Research Unit School of Forest Resources University of Georgia Athens, GA 30602 Project Officer David Moran U.S. Fish and Wildlife Service National Wetlands Research Center 1010 Gause Boulevard Slidell, LA 70458 Performed for U.S. Army Corps of Engineers Coastal Ecology Group Waterways Experiment Station Vicksburg, MS 39180 and U.S. Department of the Interior Fish and Wildlife Service Research and Development National Wetlands Research Center Washington, DC 20240 DISCLAIMER The mention of trade nanes in this report does not constitute endorsement nor recommendation for use by the U.S. Fish and Wildlife Service or Federal Government. This series may be referenced as follows: U.S. Fish and Wildlife Service. 1983-19 Species profiles: life histories and environmental requirements of coastail fishes and invertebrates. U.S. Fish Wildl. Serv. Biol. Rep. 82(11). U.S. Army Corps of Engineers, TR EL-82-4. This profile may be cited as follows: Hill, J., D.L. Fowler, and M.J. Van Den Avyle. 1989. Species profiles: life histories and environmental requirements of coastal fishes and invertebrates (Mid-Atlantic)--Blue crab. -

An Evaluation of the Effects of Blue Crab (Callinectes Sapidus) Behavior on the Efficacy of Abcr Pots As a Tool for Estimating Population Abundance
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by College of William & Mary: W&M Publish W&M ScholarWorks VIMS Articles 2011 An Evaluation Of The Effects Of Blue Crab (Callinectes Sapidus) Behavior On The Efficacy Of abCr Pots As A Tool For Estimating Population Abundance Samuel Kersey Sturdivant Virginia Institute of Marine Science KL Clark Follow this and additional works at: https://scholarworks.wm.edu/vimsarticles Part of the Aquaculture and Fisheries Commons Recommended Citation Sturdivant, Samuel Kersey and Clark, KL, "An Evaluation Of The Effects Of Blue Crab (Callinectes Sapidus) Behavior On The Efficacy Of abCr Pots As A Tool For Estimating Population Abundance" (2011). VIMS Articles. 552. https://scholarworks.wm.edu/vimsarticles/552 This Article is brought to you for free and open access by W&M ScholarWorks. It has been accepted for inclusion in VIMS Articles by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. 48 Abstract—Crab traps have been used An evaluation of the effects extensively in studies on the popula- tion dynamics of blue crabs to provide of blue crab (Callinectes sapidus) estimates of catch per unit of effort; however, these estimates have been behavior on the efficacy of crab pots determined without adequate consid- as a tool for estimating population abundance eration of escape rates. We examined the ability of the blue crab (Callinectes sapidus) to escape crab pots and the S. Kersey Sturdivant (contact author)1 possibility that intraspecific crab Kelton L. Clark2 interactions have an effect on catch rates. -

11 Shields FISH 98(1)
139 Abstract.–On the eastern seaboard of Mortality and hematology of blue crabs, the United States, populations of the blue crab, Callinectes sapidus, experi- Callinectes sapidus, experimentally infected ence recurring outbreaks of a parasitic dinoflagellate, Hematodinium perezi. with the parasitic dinoflagellate Epizootics fulminate in summer and autumn causing mortalities in high- Hematodinium perezi* salinity embayments and estuaries. In laboratory studies, we experimentally investigated host mortality due to the Jeffrey D. Shields disease, assessed differential hemato- Christopher M. Squyars logical changes in infected crabs, and Department of Environmental Sciences examined proliferation of the parasite. Virginia Institute of Marine Science Mature, overwintering, nonovigerous The College of William and Mary female crabs were injected with 103 or P.O. Box 1346, Gloucester Point, VA 23602, USA 105 cells of H. perezi. Mortalities began E-mail address (for J. D. Shields): [email protected] 14 d after infection, with a median time to death of 30.3 ±1.5 d (SE). Sub- sequent mortality rates were greater than 86% in infected crabs. A relative risk model indicated that infected crabs were seven to eight times more likely to Hematodinium perezi is a parasitic larger, riverine (“bayside”) fishery; die than controls and that decreases in total hemocyte densities covaried signif- dinoflagellate that proliferates in it appears most detrimental to the icantly with mortality. Hemocyte densi- the hemolymph of several crab spe- coastal (“seaside”) crab fisheries. ties declined precipitously (mean=48%) cies. In the blue crab, Callinectes Outbreaks of infestation by Hema- within 3 d of infection and exhibited sapidus, H. perezi is highly patho- todinium spp. -

Prevalence of Blue Crab (Callinectes Sapidus) Diseases, Parasites, And
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2014 Prevalence of Blue Crab (Callinectes sapidus) Diseases, Parasites, and Symbionts in Louisiana Holly Rogers Louisiana State University and Agricultural and Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Part of the Environmental Sciences Commons Recommended Citation Rogers, Holly, "Prevalence of Blue Crab (Callinectes sapidus) Diseases, Parasites, and Symbionts in Louisiana" (2014). LSU Master's Theses. 3071. https://digitalcommons.lsu.edu/gradschool_theses/3071 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. PREVALENCE OF BLUE CRAB (CALLINECTES SAPIDUS) DISEASES, PARASITES, AND SYMBIONTS IN LOUISIANA A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Master of Science in The School of Renewable Natural Resources by Holly A. Rogers B.S., University of Cincinnati, 2011 August 2014 ACKNOWLEDGMENTS I would like to thank my major professor, Dr. Julie Anderson, for selecting me for this assistantship and research project and for teaching more than I ever wanted to know about blue crabs. I would also like to thank Dr. Bill Kelso for his advice and instruction, particularly on scientific writing. I owe thanks to Dr. John Hawke for his guidance on crab and aquatic diseases and Dr. Sabrina Taylor for her helpful PCR advice. -

Infection by a Hematodinium-Like Parasitic Dinoflagellate Causes Pink
Journal of Invertebrate Pathology 79 (2002) 179–191 www.academicpress.com Infection by a Hematodinium-like parasitic dinoflagellate causes Pink Crab Disease (PCD) in the edible crab Cancer pagurus G.D. Stentiford,a,* M. Green,a K. Bateman,a H.J. Small,b D.M. Neil,b and S.W. Feista a Centre for Environment, Fisheries and Aquaculture Science (CEFAS) Weymouth Laboratory, Barrack Road, The Nothe, Weymouth, Dorset DT4 8UB, UK b Division of Environmental and Evolutionary Biology, Graham Kerr Building, University of Glasgow, Glasgow G12 8QQ, Scotland, UK Received 30 October 2001; accepted 2 May 2002 Abstract The edible crab (Cancer pagurus) supports a large and valuable fishery in UK waters. Much of the catch is transported live to continental Europe in specially designed live-well (‘vivier’) vehicles. During the winter of 2000/2001, many trap-caught crabs from Guernsey, Channel Islands, UK, were reportedly moribund and pink in colour. These crabs generally died before and during vivier transportation. We provide histological, immunological, and molecular evidence that this condition is associated with infection by a Hematodinium-like dinoflagellate parasite similar to that previously reported in C. pagurus and to an infection causing seasonal mass mortalities of the Norway lobster (Nephrops norvegicus). Pathologically, every altered host bore the infection, which was charac- terised by very large numbers of plasmodial and vegetative stages in the haemolymph and depletion of reserve cells in the he- patopancreas. Due to the hyperpigmentation of the carapace and appendages, we have called this infection ‘Pink Crab Disease’ (PCD). Similar Hematodinium infections cause ‘Bitter Crab Disease’ in tanner and snow crabs, which has had a negative effect on their marketability. -

Callinectes Sapidus) Dredged from Chesapeake Bay
Diseases, Parasites, and Symbionts of Blue Crabs (Callinectes sapidus) Dredged from Chesapeake Bay Gretchen A. Messick Journal of Crustacean Biology, Vol. 18, No. 3. (Aug., 1998), pp. 533-548. Stable URL: http://links.jstor.org/sici?sici=0278-0372%28199808%2918%3A3%3C533%3ADPASOB%3E2.0.CO%3B2-I Journal of Crustacean Biology is currently published by The Crustacean Society. Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/about/terms.html. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/journals/crustsoc.html. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. The JSTOR Archive is a trusted digital repository providing for long-term preservation and access to leading academic journals and scholarly literature from around the world. The Archive is supported by libraries, scholarly societies, publishers, and foundations. It is an initiative of JSTOR, a not-for-profit organization with a mission to help the scholarly community take advantage of advances in technology. For more information regarding JSTOR, please contact [email protected]. http://www.jstor.org Tue Dec 4 16:44:38 2007 JOURNAL OF CRUSTACEAN BIOLOGY, 18(3): 533-548, 1998 DISEASES, PARASITES, AND SYMBIONTS OF BLUE CRABS (CALLINECTES SAPIDUS) DREDGED FROM CHESAPEAKE BAY Gretchen A. -

First Record of a Fertilized Female Blue Crab
Aquatic Invasions (2010) Volume 5, Issue 2: 215-218 This is an Open Access article; doi: 10.3391/ai.2010.5.2.15 Open Access © 2010 The Author(s). Journal compilation © 2010 REABIC Short communication First record of a fertilized female blue crab, Callinectes sapidus Rathbun, 1896 (Crustacea: Decapoda: Brachyura), from the German Wadden Sea and subsequent secondary prevention measures Stefan Nehring1* and Uilke van der Meer2 1Federal Agency for Nature Conservation, Konstantinstraße 110, 53179 Bonn, Germany 2National Park House, Wadden Sea of Lower Saxony, Oll Deep 7, 26553 Dornumersiel, Germany E-mail: [email protected] (SN), [email protected] (UVDM) * Corresponding author Received: 22 October 2009 / Accepted: 10 December 2009 / Published online: 15 January 2010 Abstract A single female specimen of the blue crab Callinectes sapidus Rathbun, 1896 was captured by a fisherman on 26 May 2008 in the East Frisian Wadden Sea, National Park of Lower Saxony, Germany. The specimen was transferred to a public marine aquarium in Dorumersiel. On 9 June 2008 the female extruded masses of fertilized eggs. Due to nature conservation efforts in this national park, management instructions are implemented by the appropriate authority for further dealing with the captured specimen and its potential offspring. This is the second record of a mature female and the first record of a fertilized female blue crab from German coastal waters of the North Sea. Key words: Callinectes sapidus, Crustacea, alien species, North Sea, Germany, management A single fertilized female specimen of the Ame- specimens of the balanoid Balanus crenatus rican blue crab, Callinectes sapidus Rathbun, Bruguière, 1789 (Figure 1) which indicates a 1896, was caught by a shrimp fisherman using a considerable period of time since its last molt shrimp beam trawl on 26 May 2008 while fishing took place. -
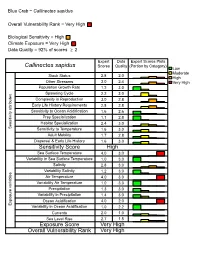
Callinectes Sapidus
Blue Crab − Callinectes sapidus Overall Vulnerability Rank = Very High Biological Sensitivity = High Climate Exposure = Very High Data Quality = 92% of scores ≥ 2 Expert Data Expert Scores Plots Callinectes sapidus Scores Quality (Portion by Category) Low Moderate Stock Status 2.8 2.0 High Other Stressors 3.0 2.4 Very High Population Growth Rate 1.2 3.0 Spawning Cycle 2.2 3.0 Complexity in Reproduction 3.0 2.8 Early Life History Requirements 2.8 2.8 Sensitivity to Ocean Acidification 1.6 2.6 Prey Specialization 1.1 2.8 Habitat Specialization 2.4 3.0 Sensitivity attributes Sensitivity to Temperature 1.6 3.0 Adult Mobility 1.7 2.8 Dispersal & Early Life History 1.6 3.0 Sensitivity Score High Sea Surface Temperature 4.0 3.0 Variability in Sea Surface Temperature 1.0 3.0 Salinity 2.8 3.0 Variability Salinity 1.2 3.0 Air Temperature 4.0 3.0 Variability Air Temperature 1.0 3.0 Precipitation 1.3 3.0 Variability in Precipitation 1.4 3.0 Ocean Acidification 4.0 2.0 Exposure variables Variability in Ocean Acidification 1.0 2.2 Currents 2.0 1.0 Sea Level Rise 2.7 1.5 Exposure Score Very High Overall Vulnerability Rank Very High Blue Crab (Callinectes sapidus) Overall Climate Vulnerability Rank: Very High (42% certainty from bootstrap analysis). Climate Exposure: Very High. Three exposure factors contributed to this score: Ocean Surface Temperature (3.9), Air Temperature (4.0) and Ocean Acidification (4.0). Blue Crab use estuarine and ocean habitats during different portions of the life cycle.