Blue Crab (Callinectes Sapidus) Ecology: Review and Discussion Regarding Tisbury Great Pond
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SUMMER CAMPS Bprcamps.Org • 2021
Boulder Parks & Recreation SUMMER CAMPS BPRcamps.org • 2021 REGISTER TODAY! - Camps fill up fast! Choose from 125+ camp sessions for youth ages 4-17! RESERVOIR CAMPS • GOATS & GARDENS • KIDZ KAMP • GYMNASTICS • DRAMA • POTTERY • SPORTS LEGO ENGINEERING • SAILING • BIKING & MORE! Camp Planner Registration: 303-413-7270 or BPRcamps.org, unless otherwise noted. Page # Page Camp Day/Time Ages # of Days Fee (R) = Resident (N) = Non-Resident May 31-June 4 31-June May June 7-11 June 14-18 June 21-25 2 June28-July July 5-9 July 12-16 July 19-23 July 26-30 2-6 Aug. 9-13 Aug. REZ CAMP provided by the City of Boulder 4 Rez Camp M-Th, 8:30am-4pm 7-14 4 $316(R)/$352(N) • • • • • • • GOATS & GARDENS provided through the City of Boulder 5 Goats & Gardens M-F, 8:30am-3:30pm 5-11 5 $350(R)/$385(N) • • • • • • • • • KIDZ KAMP provided by the City of Boulder 6 Kidz Kamp East M-F, 8:30am-4pm 5-11 5 $567-$700 (2wks) • • • • • GYMNASTICS provided by the City of Boulder 7 Summer Recreational Gymnastics M-F, 9am-12pm 5+ 5 $216(R)/$268(N) • 7 Summer Recreational Gymnastics M/W/F, 9am-10:30am 3-4 3 $65(R)/$81(N) • DANCE provided by our partner Kinesis Dance 7 Dancing Days M-Th, 9am-12pm 3 4 $175 • • 7 All About Dance Camp M-Th, 9am-1 & 1-5pm 6-10 4 $225 • • DRAMA CAMP provided by our partner Miss Joanie at MissJoanieDrama.com 8 Drama M-F, 8:30am-4:30pm 4-11 4-5 $280-$350 • • • • • • • • • • • POTTERY CAMPS provided by our partner Studio Arts Boulder 9 Wheel-throwing M-F, 9-12pm or M-F, 1-4pm 8-11 5 $240 • • • • • • • 9 Wheel-throwing M-F, 9-12pm or M-F, 1-4pm -

2016 Parent Handbook
Sagamore Junior Sailing Program 2016 PARENT HANDBOOK Revised June 10, 2016 Sagamore Junior Sailing Program / LuHi Parent Handbook LuHi, welcome to the 2016 Sagamore Junior Sailing Program! These pages are meant to be a parent’s and future junior sailor’s guide in navigating the child’s way through the rewarding sport of junior sailing. In these pages you will find details on recommended equipment for the children to bring to the program, goals the program will focus for your child and the expectations of the parents themselves. The LuHi Summer Sailing Program is for Grades 4 thru 9. Sagamore Junior Sailing Mission The Sagamore Junior Sailing (SJS) Fleet’s Mission is to provide a fun and educational program for youth interested in sailing and racing. We seek to impart a love of sailing as a life sport while providing the fundamental skills necessary for participants to advance in the sport of sailing as far as their desire, skill and hard work may take them. Our instructors shall provide a curriculum with the most current and effective techniques to create skilled and confident youths who will respect others, care for their equipment and be willing to help fellow sailors on and off the water. Our sailors will be expected to conduct themselves in the Corinthian Spirit during practice and in competition. All Junior Fleet sailors, whether their interest be in cruising or racing, should emerge with an enhanced sense of self-reliance, knowledge of seamanship and a solid foundation in the sport of sailing. An Overview of Junior Sailing Junior Sailing can be like any other full time sport such as baseball, soccer or tennis – what a child puts into it will determine what a child will get out of the program. -
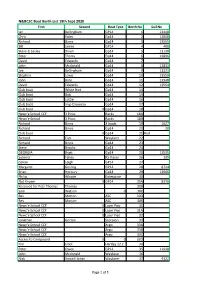
N&BCSC Boat Berth List 19Th Sept 2020
N&BCSC Boat Berth List 19th Sept 2020 First Second Boat Type Berth No Sail No Ian Bellingham GP14 1 13440 Chris Yates Gp14 2 13868 Richard Binns Gp14 3 13555 Bill James GP14 4 408 Steve & Lesley Dixon Gp14 5 13138 Peter Thoms Gp14 6 13896 David Edwards Gp14 7 John Mcdonald Gp14 8 12831 Joe Bellingham Gp14 9 13321 Stephen Lewis Gp14 10 13559 John Bate Gp14 11 13948 David Edwards Gp14 12 13554 Club boat White Riot Gp14 14 Club boat Bob Gp14 15 Club boat Lottie Gp14 16 Club boat Insp Clouseau Gp14 17 Club boat 0 Gp14 18 Newc'e School CCF 3 Picos Racks 18A Newc'e School 3 Picos Racks 18B Richard Binns 3 boats 19 1627 Richard Binns Gp14 20 20 Club boat 0 Gp14 21 Red Richard Fish Wayfarer 22 Richard Binns Gp14 23 Steve Brooks Gp14 24 GEORGIA Bratt Gp14 25 12535 Suteera Fahey RS Vision 26 195 Ceiran Leigh GP14 27 Margaret Gosling GP14 28 8724 Brian Horbury Gp14 29 13066 Philip Whaite Enterprise 30 Not Known 0 GP14 30A 8376 Reserved for Pete Thomas Thomas 30B Sam Watson 0 30C Bev Morton ASC 30D Bev Morton ASC 30E Newc'e School CCF 0 Laser Pico 31 Newc'e School CCF 0 Laser Pico 31A Newc'e School CCF 0 Laser Pico 32 Jonathan Gorton Scorpion 33 Newc'e School CCF Argo 33A Newc'e School CCF Argo 33B Newc'e School CCF Argo 33C Access to Compound 0 0 33D Tim Elliot Hartley 12.2 34 Peter Owen GP14 35 11940 John Mcdonald Wayfarer 36 Nick Howell-Jones Wayfarer 37 9322 Page 1 of 5 Sean Barton Gp14 38 Martin Kirby Gp14 39 18673 Sam Barker Gp14 40 10459 Mark Price GP14 41 Gill Fox Gp14 42 6244 Andrew Dulla Gp14 43 Tracy Haden Gp14 44 11643 Rob Barlow GP14 -

Muck Removal Efficiency Plus Biological and Chemical Responses/Improvements After Dredging (Subtask 2)
Impacts of Environmental Muck Dredging, Florida Institute of Technology, 2017-2018, November 2019 Muck Removal Efficiency plus Biological and Chemical Responses/Improvements after Dredging (Subtask 2) Kevin B. Johnson, Jonathan M. Shenker, John H. Trefry, Co-Principal Investigators [email protected] (321-674-7186); [email protected] (321-674-8145); [email protected] (321-258-4174) Austin L. Fox, Collaborating Scientist [email protected] (321-674-7463) Indian River Lagoon Research Institute Florida Institute of Technology 150 West University Boulevard Melbourne, Florida 32901 Original Submission, November 2019 Revised Final Submission, July 2020 i Impacts of Environmental Muck Dredging, Florida Institute of Technology, 2017-2018, November 2019 Impacts of Environmental Muck Dredging 2017‒2018 Muck Removal Efficiency plus Biological and Chemical Responses/Improvements after Dredging (Subtask 2) Final Project Report Submitted to Brevard County Natural Resources Management Department 2725 Judge Fran Jamieson Way, Building A, Room 219 Viera, Florida 32940 Funding provided by the Florida Legislature as part of DEP Grant Agreement No. NS005 – Brevard County Muck Dredging Co-Principal Investigators: Kevin B. Johnson, Jonathan M. Shenker, John H. Trefry Austin L. Fox, Collaborating Scientist Indian River Lagoon Research Institute Florida Institute of Technology 150 West University Boulevard Melbourne, Florida 32901 Original Submission, November 2019 Revised Final Submission, July 2020 ii Impacts of Environmental Muck Dredging, Florida Institute of Technology, 2017-2018, November 2019 Muck Removal Efficiency plus Biological and Chemical Responses/Improvements after Dredging (Subtask 2) Kevin B. Johnson, Jonathan Shenker, John H. Trefry, Austin L. Fox Florida Institute of Technology, Melbourne, Florida 32901 Executive Summary Removal of fine-grained, organic-rich sediments is an integral part of restoring the Indian River Lagoon (IRL) to a healthy ecosystem. -

Blue Crab (Callinectes Sapidus) Use of the Ship/Trinity/Tiger Shoal
ACCESS NUMBER: 32806-35161 STUDY TITLE: Ship Shoal: Sand, Shrimp, and Seatrout REPORT TITLE: Blue Crab (Callinectes sapidus) Use of the Ship/Trinity/Tiger Shoal Complex as a Nationally Important Spawning/Hatching/Foraging Ground: Discovery, Evaluation, and Sand Mining Recommendations based on Blue Crab, Shrimp, and Spotted Seatrout Findings CONTRACT NUMBER: 1435-01-04-CA-32806-35161 (M05AZ10660) SPONSORING OCS REGION: Gulf of Mexico APPLICABLE PLANNING AREA: Central FISCAL YEARS OF PROJECT FUNDING: 2004-2009 COMPLETION DATE OF REPORT: December 2009 COSTS: FY 2004-2009, $145,778; CUMMULATIVE PROJECT COSTS: $145,778 PROJECT MANAGER: R. E. Condrey AFFILIATION: Louisiana State University, Department of Oceanography and Coastal Sciences, School of the Coast and Environment ADDRESS: Energy, Coast, and Environment Building, Louisiana State University, Baton Rouge, LA 70803 PRINCIPAL INVESTIGATOR: R.E. Condrey KEY WORDS: Gulf of Mexico, Ship Shoal, Trinity Shoal, Tiger Shoal, Louisiana, sand mining, blue crab, white shrimp, brown shrimp, spotted seatrout, condition factor, fecundity, spawning grounds, coastal restoration BACKGROUND: The Minerals Management Service (MMS) is addressing the recent demand for U.S. continental shelf sand resources for coastal erosion management, a critical challenge to Louisiana’s ecosystems and economies. Louisiana considers barrier island restoration as a promising way to combat wetland loss, with sand mined from Ship Shoal as the most feasible sediment source. Additional sand resources on Trinity and Tiger Shoals are also being considered. Since this area supports major demersal fisheries on white and brown shrimp (Litopenaeus setiferus and Farfantepenaeus aztecus), the present study was conducted as part of a larger effort to understand the sand-mining ecology of the Ship/Trinity/Tiger Shoal Complex (STTSC). -

The Lazu Sheet
d EJffi, R@\€E €F#*r ^#lW#ffi The LazU Sheet :: The official newsletter of the Lake Mission Viejo Yacht Club April "98w TIwVoUage Continu,es by John Robrnson (Editor) Welcome aboard for our next (actually it is the phone booth rn Chili Cook-off instaltment of "The Lazy front of Carl's Jr. on [aPaz) we'll (April 19th Regatta) Sheet". I would like to take try to put this month's copy out Are you ready ??? This is this space to thank all of you with some good information in a a call to all you chili cooks who: a) read the newsletter fun format. Look for more poetry (?) who think that you have the and b) who had kind things from our Mystery Poet (I still don't hottest stuff goin'. If you to say to me about my efforts. have a clue who it is) as well as the want to enter your rump- I will even take the message official LI\AIYC 1998 Sailing and burnin', hole-scorchin', fire- received by E-mail from Social Calendar. belchin', mouth-of-hell style "Sailnut@juno. com" as a recipe then by all means complement (yes, I'm sure that I invite any and all members to join call up little ol'Linda it WILL get better as time goes in the fun by subrniuing articles, Schaffner and tell her ! bv). jokes (clean one's), pictures, things Numbers will be given for sale (or sail) to me by fax (714) to entrants to €rssure an When I told Roger that I would 546-3733, hand written on a lunch impartial jury and we will be happy to do the newsletter, bag, or E-mailed (LIvIVYCNEWS eat and judge. -

Meet the Competitors: Annapolis YC Double-Handed Distance Race
Meet the Competitors: Annapolis YC Double-handed Distance Race R.J. Cooper & Courtney Cooper Cumberland are a brother and sister team from Oxford, Maryland and Panama City, Florida. They have sailed together throughout their youth as well as while on the Sailing Team for the University of Florida. The pair has teamed up for a bid to represent the United States and win gold at the 2024 Olympics in Paris. They will be sailing Tenacious owned by AYC member Carl Gitchell. Sail #501 Erik Haaland and Andrew Waters will be sailing the new Italia Yachts 9.98 sport boat named Vichingio (Viking). Erik Haaland is the Sales Director for Italia Yachts USA at David Walters Yachts. He has sailed his entire life and currently races on performance sport boats including the Farr 30, Melges 32 and J70. Andrew Waters is a Sail and Service Consultant at Quantum Sails in Annapolis. His professional sailing career began in South Africa and later the Caribbean and includes numerous wins in large regattas. Sail #17261 Ethan Johnson and Cat Chimney have sailing experience in dinghies, foiling skiffs, offshore racers and mini-Maxis. Ethan, a Southern Maryland native now living in NY is excited to be racing in home waters. Cat was born on Long Island, NY but spent time in Auckland, New Zealand. She has sailed with Olympians, America’s Cup sailors and Volvo Ocean Race sailors. Cat is Technical Specialist and Rigger at the prestigious Oakcliff Sailing where Ethan also works as the Training Program Director. Earlier this year Cat and Ethan teamed up to win the Oakcliff Double-handed Melges 24 Distance Race. -

Wind Wars Sailing the North Channel to Meldrum Bay
Volume XX No. 5 Oct/Nov/Dec 2009 Wind Wars Sailing the North Channel to Meldrum Bay Lake of the Woods - LOWISA 44 Team Racing at the Club Level Boat Smart: EPIRB Over 500 New and Used Boats IT’S TIME FOR THAT BOAT 38TH MINNEAPOLIS BOAT SHOW ® January 20—24, 2010 Minneapolis Convention Center Shop, compare and save on new boats and the latest in boating gear. Affordability Pavilion—Shop boats less than $250/month. SailFest—A dedicated sailing seminar series. Boat Show tickets make a great holiday gift! On sale December 7. Pre-shop the show and more at MinneapolisBoatShow.com 38TH ANNUAL Produced by le Islands Adventure Be Apost gins He Your re! Lake Minnetonka’s Premier Sailboat Marina Now Reserving Slips for the 2010 Sailing Season! State Of The Art Marina In An Extremely Weather-Safe Harbor Call About Our New Customer Slips Available for 2010! Specials 1 ½ miles south of Bayfield on Highway 13 P.O. Box 716 • Bayfield, Wisconsin 54814 952-474-0600 Toll Free: 877-841-3900 www.pikesbaymarina.com [email protected] S A I L I N G S C H O O L Safe, fun, learning Safe, fun, learning . Caribbean School of British Virgin Islands Learning Adventures in the best cruising grounds in the Caribbean. the Year ASA One-Week Courses in the Caribbean: Basic Cruising/Bareboat Charter, Cruising Multihull, Gold Standard Advanced Coastal Cruising, Fun only/Flotilla (No Experience). February - April, 2010 • Offshore Advanced Coastal Cruising: Tortola, Bonaire, Puerto Rico, Spanish Virgins and More! February & April, 2010 (Over 400 nm each way) • Sail & Dive - Small Groups Aboard Catamarans. -

Dimorphism and the Functional Basis of Claw Strength in Six Brachyuran Crabs
J. Zool., Lond. (2001) 255, 105±119 # 2001 The Zoological Society of London Printed in the United Kingdom Dimorphism and the functional basis of claw strength in six brachyuran crabs Steve C. Schenk1 and Peter C. Wainwright2 1 Department of Biological Science, Florida State University, Tallahassee, Florida 32306, U.S.A. 2 Section of Evolution and Ecology, University of California, Davis, CA 95616, U.S.A. (Accepted 7 November 2000) Abstract By examining the morphological basis of force generation in the chelae (claws) of both molluscivorous and non-molluscivorous crabs, it is possible to understand better the difference between general crab claw design and the morphology associated with durophagy. This comparative study investigates the mor- phology underlying claw force production and intraspeci®c claw dimorphism in six brachyuran crabs: Callinectes sapidus (Portunidae), Libinia emarginata (Majidae), Ocypode quadrata (Ocypodidae), Menippe mercenaria (Xanthidae), Panopeus herbstii (Xanthidae), and P. obesus (Xanthidae). The crushers of the three molluscivorous xanthids consistently proved to be morphologically `strong,' having largest mechan- ical advantages (MAs), mean angles of pinnation (MAPs), and physiological cross-sectional areas (PCSAs). However, some patterns of variation (e.g. low MA in C. sapidus, indistinguishable force generation potential in the xanthids) suggested that a quantitative assessment of occlusion and dentition is needed to understand fully the relationship between force generation and diet. Interspeci®c differences in force generation potential seemed mainly to be a function of differences in chela closer muscle cross- sectional area, due to a sixfold variation in apodeme area. Intraspeci®c dimorphism was generally de®ned by tall crushers with long in-levers, though O. -

Venezuela's Cordero - World Champ Again Upcoming Regattas
The Official Newsletter of the International Sunfish® Class Association venezuela's Cordero - world Champ Again Upcoming Regattas ... Midwinters Eduardo Wins his Sthl by Charlie Clifton Clearwater. FL. March 1-4, 2001 International Masters Punta Gorda, FL. March 9-11, 2001 NA/Jr. NA Championship Jr's: July 30-Aug.1 , NA's Aug . 1-4, 2001 Highland Park. IL National Masters vc of Stone Harbor. NJ June 22-24 women's NA's Center Moriches. NY. Sept (date pending) points were awarded. Johansson managed a fifth despite effecting repairs in the middle of the race. Martinborough mistook a boat recording finishes for the pin end of the finish line. He went from 5'h to 19'h before he could correct the error. Cordero managed an S'h which put him in second place, 7 points behind Oskar. Wednesday, the fourth race started in a light easterly which died and then shifted to just after the stan of Race 3 in Sarasota at the Worlds. Photo by Regatta Photography the north halfway through the race. The November 16'h, the night before the definitely the fastest junior, Diego right side of the fleet was hung out to dry, final day of the 2000 Sunfish World Zimmerman. Cordero among them. Johansson won. As Championship on Sarasota Bay, Eduardo It looked like Olcese had done it again the wind settled in for the next two races, he Cordero was calm and reflective. ''This is the in the second race as he finished first but, and Jeff Linton alternated firsts and ninths first time I haven't been nervous going into when the scores were posted, he was among while Cordero stayed in the average with the last day. -

December Channel 2002
OCTOBER 2003 TheCHANNEL Coconut Grove Sailing Club SKIPPER OF THE MONTH: 2003-2004 Officers C. J. Abell and Committee C J Abell started sailing at the CGSC when he was 9 Chairmen years old. It was a family thing. His dad, Charlie would Flag Officers take C J and his younger brother Michael to sail in an older Commodore ........................... Bud Price brothers boat, which had been sitting around the yard for Vice Commodore................ Jack Hamm quite some time. After a couple of weekends of sailing Rear Commodore...... Vladimir Stroleny they signed up for beginning lessons at the Miami Yacht Secretary ............................... Anne Platt Club. About a month later, the CGSC got its program Treasurer ..................... Jeffrey Zirulnick going again and C J began sailing in the CGSC racing and Fleet Officers summer programs. C J says that after the first week he Fleet Captain ........................Wil Bourne knew that he loved sailing. Fleet Chaplain .......... Brian C. Schofield Charlie got a small group to- Fleet Surgeon ....... Dr. Nicolaus Martens gether to begin a racing team. Board Members They would go out every weekend on both Saturday and Sunday Gonzalo Bellini Nick Martens with one of the parents on watch in a power boat. Jason Timmons, Jim Clark Felipe Mejia a past Opti Champion, became their coach. C J credits Jason for Steve Hawkins Jonathan Milley the inspiration to push himself to make and achieve goals and for Mike Lovelady Charles Rahn giving so much to the team, that he will always be remembered. Pat Gerry Marston Andrea Stringos, P.C. Downy, also became the CGSC racing coach and was instrumental in fine-tuning their skills. -

Starboard Quarter Skipper in the Spotlight Jungle on “Bat” Island
Starboard Spring Quarter March 1, 2003 Yacht Club/YMCA “Sailing Season” a Inside this issue: Grand Success (B.E.R.T.) and Coast 2 “Culture of Care.” Guard Auxiliary More than 60 have joined “Sailing Season” to make Claudia Wilcox-Boucher was able to provide the it an overwhelming suc- “Naupaka” Ameri- 2 YMCA’s Sunfish, a cess. can 25, safety boat and life- The combined effort of our Classifieds guard and Nick was club and the YMCA to able to convince mem- sponsor our first “Sailing Sunfish Fleet is Ready Skipper in the Spot- 3 bers of our club to dedicate Season” has brought both light, YMCA Class, their time and instructional organizations many new Schotte, Mauricio Barbis, 2002 Champion ability. members and introduced Stefano Barbis, John Lu- Club Site, Sunfish, 4 new sailors to the sea. The first day of “Sailing chau, Kim Magnuson, Sunfish Restoration Season” brought more sail- Kevin Horton, Jon Olson, “Sailing Season” is headed ors to the beach on that Larry and Robbyn Peck, by Nick Rees who began blustery January day than Arjun Clarry, Mac Haiku, coordinating instructors Sailing Season 5 were anticipated which Jacques Peysson, Joel and boats at the end of (cont’d), made the day exciting and Rutter and Debbie Kuntz. last year. Nick’s vision very busy for Nick and the Thank you to all Volun- was to provide an oppor- rest of the volunteer in- teers and participants. Club History, DLNR 5 tunity for everyone who structors. Can you imag- Boating had any desire to sail to For Participants: Bring ine seven Sunfish and be able to get out on Sunscreen, hat, shirt, nearly 50 people wanting to boats.