Invited Review Characterisation of Thyroid Medullary Carcinoma TT Cell
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Histogenesis of Medullary Carcinoma of the Thyroid
J. clin. Path., (1966), 19, 114 Histogenesis of medullary carcinoma of the thyroid E. D. WILLIAMS1 From the Institute ofPathology, The London Hospital SYNOPSIS Thirty-one dog thyroid tumours and 28 spontaneous rat thyroid tumours were studied histologically and the findings compared with those of a study of 67 cases of medullary carcinoma of the human thyroid. Five of the dog tumours and 24 of the rat tumours were considered to belong to the same group of tumours as medullary carcinoma, a group characterized by solid sheets or lobules of uniform cells with granular cytoplasm and without papillary or follicular differentiation. In the rat tumours it was shown that the cell of origin was the parafollicular cell and not the thyroid follicle epithelial cell. It is suggested that medullary carcinoma is also derived from a parafollicular cell and that this origin would resolve the discrepancy between the relatively good prognosis and the apparently undifferentiated structure of this tumour. It is also concluded that the whole spectrum of clinical and pathological features of medullary carcinoma makes more sense if it is considered as a parafollicular cell tumour. Medullary carcinoma of the thyroid is a distinct the other 19 tumours, two were classified as papillary and clearly defined entity, and the histological carcinomas, nine as follicular carcinomas, two as features of 67 examples are discussed in the previous anaplastic carcinomas, while one tumour showed a paper (Williams, Brown, and Doniach, 1966). The mixture of follicular carcinoma with osteochondro- survival of patients with this tumour is often pro- sarcoma. The remaining five tumours were similar longed although paradoxically the vast majority of and were made up of nests ofuniform cells, separated cases show no evidence of differentiation towards a into groups by a connective tissue stroma which was thyroid epithelial pattern. -

Endocrine Glands
Endocrine glands David Kachlík Endocrine system • one out if two regulator systems • phylogenetically older than the nervous system • regulates activity of other systems so that they could react to changing requirements of outer and inner environment (maintains homeostasis) • does not originate from anatomically similar structures • secretion into blood – possesses no ducts • nearly all organs and tissues of the human body produce a hormone Hormone • horman in Greek = to arise • chemical messanger produced by endocrine gland and transported into blood to target organs • proteins (polypeptides) – insuline • amines – adrenaline • steroids – estrogenes Clinical consequence • hormonal excess – primary gland overproduction – secondary to excess production of trophic (releasing, stimulating) substance (hormone) • hormonal deficiency – primary gland failure – secondary to lack of stimulation by trophic (releasing, stimulating) substance (hormone) – target organ resistance Endocrine glands History Thomas Wharton • 1614-1673 • Adenographia • first detailed description of glands Ernest Henry Starling • 1866-1927 • general schemes of „endocrine secretion“ • used the already exsiting word „hormones“ Endocrine system arrangement • glands • disseminated cells • neuroendocrine cells Endocrine glands – list • hypothalamus (hypothalamus) • pituitary gland (hypophysis; gl. pituitaria) • thyroid gland (glandula thyroidea) • parathyroid bodies (gll. parathyroideae) • suprarenal glands, adrenals (gll. suprarenales) • pancreatic (Langerhans‘) island (insulae -

A Case of Calcitonin Producing Adenoma of the Thyroid
Endocrinol. Japon. 1984, 31 (1), 63-70 A Case of Calcitonin Producing Adenoma of the Thyroid TAKAYA KODAMA, MASAKO TAMURA, YOSHIHARU KANAJI, YUKIO ITO, TAKAO OBARA, YOSHIHIDE FUJIMOTO AND AKIRA HIRAYAMA* Department of Endocrine Surgery and Department of Surgical Pathology*, Tokyo Women's Medical College. Ichigaya-Kawadacho, Shinjuku-ku, Tokyo 162 Abstract A thyroid tumor about 4 cm in diameter was removed from a 53-year-old female. The clinical features were those of common thyroid adenomas, such as soft, smooth-surfaced and round contour of the tumor, no calcification and no lymph node metastasis. However, microscopically, the tumor was composed of compact cell nests rather similar to paraganglioma without any follicle formation. Histochemical examination revealed its C-cell nature, such as argyrophilia and calcitonin activity, although no amyloid deposit was demonstrated. Extremely high levels of calcitonin were found in the stored serum taken pre-operatively, but serum carcinoembryonic antigen levels were within normal limits. The calcitonin level dropped to the normal range soon after the operation, indicating the absence of residual tumorous tissues. Thus, the tumor behaves as an adenoma with a C-cell nature. Interestingly enough, the benign counterpart of medullary carcinoma of the thyroid seems to be quite rare in humans, and no similar tumors have ever been reported. Variations of C-cell tumors will be discussed in relation to their production and secretion of carcinoembryonic antigen. Medullary carcinoma of the thyroid hormones, confirming the C-cell as the origin (MCT) was first regarded as a clinical entity of MCT. by Hazard et al. (1959), recognizing a group A wide variation of morphological futures of solid non-follicular tumors with com- and prognosis of MCT had already been paratively good prognosis among apparently pointed out by Williams et al. -
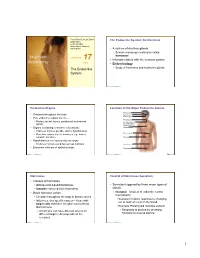
The Endocrine System
PowerPoint® Lecture Slides The Endocrine System: An Overview prepared by Leslie Hendon University of Alabama, Birmingham • A system of ductless glands • Secrete messenger molecules called hormones C H A P T E R 17 • Interacts closely with the nervous system Part 1 • Endocrinology The Endocrine • Study of hormones and endocrine glands System Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Endocrine Organs Location of the Major Endocrine Glands Pineal gland • Scattered throughout the body Hypothalamus Pituitary gland • Pure endocrine organs are the … Thyroid gland • Pituitary, pineal, thyroid, parathyroid, and adrenal Parathyroid glands glands (on dorsal aspect of thyroid gland) • Organs containing endocrine cells include: Thymus • Pancreas, thymus, gonads, and the hypothalamus Adrenal glands • Plus other organs secrete hormones (eg., kidney, stomach, intestine) Pancreas • Hypothalamus is a neuroendocrine organ • Produces hormones and has nervous functions Ovary (female) Endocrine cells are of epithelial origin • Testis (male) Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Figure 17.1 Hormones Control of Hormones Secretion • Classes of hormones • Amino acid–based hormones • Secretion triggered by three major types of • Steroids—derived from cholesterol stimuli: • Basic hormone action • Humoral—simplest of endocrine control mechanisms • Circulate throughout the body in blood vessels • Secretion in direct response to changing • Influences only specific tissues— those with ion or nutrient levels in the blood target cells that have receptor molecules for that hormone • Example: Parathyroid monitors calcium • A hormone can have different effects on • Responds to decline by secreting different target cells (depends on the hormone to reverse decline receptor) Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. -

Anatomy of Endocrine System
Anatomy of Endocrine system Introduction, Pituitary gland and Thyroid gland Prepared by Dr. Payal Jain Endocrine System I. Introduction A. Considered to be part of animals communication system 1. Nervous system uses physical structures for communication 2. Endocrine system uses body fluids to transport messages (hormones) II. Hormones A. Classically, hormones are defined as chemical substances produced by ductless glands and secreted into the blood supply to affect a tissue distant from the gland, but now it is understood that hormones can be produced by single cells as well. 1. epicrine a. hormones pass through gap junctions of adjacent cells without entering extracellular fluid 2. paracrine a. hormones diffuse through interstitial fluid (e.g. prostaglandins) 3. endocrine a. hormones are delivered via the bloodstream (e.g. growth hormone Different endocrine glands with cell Organ Division arrangement Cell arrangement/morphology Hormone Hypophysis Adenohypophysis Pars distalis Cells in cords around large-bore capillaries: Acidophils Growth hormone, prolactin Basophils ACTH, TSH, FSH, LH Pars intermedia Mostly basophilic cells around ACTH, POMC cystic cavities Pars tuberalis Narrow sleeve of basophilc cells LH around infundibulum Neurohypophysis Pars nervosa Nerve fibers and supporting cells Oxytocin and (pituicytes) vasopressin (produced in hypothalamus) Infundibulum Nerve fibers (traveling from hypothalamus to pars nervosa) Pancreas Islet of Langerhans Irregularly arranged cells with Insulin, glucagon many capillaries Follicles: Simple -

Internet Journal of Medical Update 2012 July;7(2):54-56
Internet Journal of Medical Update 2012 July;7(2):54-56 Internet Journal of Medical Update Journal home page: http://www.akspublication.com/ijmu Case Report Medullary carcinoma of the thyroid - an unusual case of hyalinizing trabecular adenoma - like variant (encapsulated) Vidya Bhat*ᴪ MD and Madhusmita Jena** MD *Assistant Professor, **Associate Professor, MVJ Medical College and Research Hospital, Bangalore, Karnataka, India (Received 10 September 2011 and accepted 12 January 2012) ABSTRACT: Medullary thyroid carcinoma is a neoplasm occurring in sporadic and familial patterns. A rare variant of medullary thyroid carcinoma shows microscopic features similar to hyalinizing trabecular adenoma of thyroid. Detection of this variant requires a high index of suspicion and immunohistochemical confirmation by calcitonin positivity. We present a 36 years old female patient with a thyroid nodule which, on microscopy, displayed an encapsulated tumor with elongated cells arranged in trabecular pattern separated by hyalinized fibrous septae simulating a hyalinizing trabecular adenoma. Also present were spindle cells arranged in an organoid fashion. Most of the cells showed salt and pepper chromatin pattern. The lesion was negative for amyloid but showed diffuse calcitonin positivity indicative of a tumor of C-cell origin i.e. medullary carcinoma of thyroid – hyalinizing trabecular variant. KEY WORDS: Thyroid; Medullary carcinoma; Hyalinizing trabecular adenoma INTRODUCTIONᴪ a solitary nodule measuring 4 x 4 cm in the right lobe. No lymph nodes were palpable. The patient Most Medullary Thyroid Carcinomas (MTC) can was clinically diagnosed as nodular goitre. Her be diagnosed by their unique morphological and laboratory investigations revealed a euthyroid state. immunohistochemical features. However several There was no family history of thyroid swelling. -

L2,3- Thyroid and Parathyroid Glands
Thyroid and Parathyroid glands Red: important. Black: in male|female slides. Gray: notes|extra. Editing file Ø OBJECTIVES o Describe the histological structure of THYROID GLAND o Identify and correlate between the different ENDOCRINE CELLS in THYROID GLAND and THEIR FUNCTIONS o The microscopic structure of the PARATHYROID GLAND o The functional structure of the PARATHYROID CELLS Histology team 437 | Endocrine block | Lecture two & three Ø Thyroid Gland THYROID GLAND STROMA PARENCHYMA 1- Capsule: dense irregular collagenous C.T. THYROID FOLLICLES: 2- Septa (Interlobular septa) Are the structural and functional units of the 3- Reticular fibers: thyroid gland. Thin C.T., composed mostly of reticular L/M: fibers with rich capillary plexus surrounds 1- Simple cuboidal epithelium: each thyroid follicle. a- Follicular cells. Highly vascular to supply the gland with amino acids and Iodine b- Parafollicular cells. 2- Colloid: central colloid-filled lumen. Acidophil gel like material N.B. Each follicle is surrounded by thin basal lamina. Parafollicular cells Follicular cells Colloid Histology team 437 | Endocrine block | Lecture two & three FOLLICULAR (PRINCIPAL) CELLS PARAFOLLICULAR CELLS 99.9% of the cells (CLEAR CELLS) (C-CELLS) 0.1% of the cells L/M -Simple cuboidal cells -Pale-stained cells (Clear Cells). -Round nucleus with prominent nucleoli. -Are found singly or in clusters in between -Basophilic cytoplasm. the follicular cells. -Apical surface reaches -Their apices do not reach the lumen of the lumen of the follicle. the lumen of the thyroid -Are larger than follicular cells follicle. (2-3 times). -Only 0.1% of the epithelial cells. -Have round nucleus E/M - Mitochondria. -

Cytomorphological Features of Medullary Thyroid Carcinoma
WJOES Sushila Jaiswal et al 10.5005/jp-journals-10002-1229 ORIGINAL ARTICLE Cytomorphological Features of Medullary Thyroid Carcinoma: An Analysis based on 41 Ultrasound- guided Fine-needle Aspiration Specimens 1Sushila Jaiswal, 2Raghunandan Prasad, 3Farhana Siddiqui, 4Hira Lal, 5Neha Nigam, 6Azfar Neyaz 7Sabaretnam Mayilvaganan, 8Gyan Chand, 9Amit Agarwal ABSTRACT to eccentric nuclei were present in 39% of specimens, while 13% of specimens showed mainly eccentric nuclei. In all the Introduction: Medullary thyroid carcinoma (MTC) is a malig- specimens, neuroendocrine type of salt and pepper-like mor- nant tumor of thyroid gland showing parafollicular or C-cell phology was present which is best seen with Papanicolaou differentiation. (Pap) stain. Similarly, cytoplasm showed granules in 78% and fine vacuoles in 29% of specimens. Amyloid appreciated as Aims and objectives: The current study was undertaken to flecks or aggregates of amorphous pink or gray color was seen evaluate safety of ultrasound-guided thyroid fine-needle aspira- in 37% of specimens. No mitosis was noted in any specimen, tion cytology (FNAC) and to assess cytomorphological features while necrosis was seen in only in one specimen. of MTC in FNA specimens from 28 patients. Conclusion: Ultrasound-guided FNAC is a fairly accurate, The study was performed by retro- Materials and methods: relatively safe, rapid, and simple tool for preoperative diagnosis spectively reviewing the clinical and pathological records of of thyroid malignancies. Although the cytological features of MTC cases managed at our institute. MTC are well described, different patterns may pose a diag- Results: The patients included 18 males and 10 females with nostic difficulty. a mean age of 45.3 years; 24 specimens were taken from Keywords: Fine-needle aspiration cytology, Fine-needle aspi- thyroid, 15 from cervical lymph nodes and one each from liver ration cytology thyroid, Medullary thyroid carcinoma, Thyroid space occupying lesion and chest wall nodule. -

Chapter Twenty
Chapter 20 Outline • Endocrine Glands and Hormones • Hypothalamic Control of the Endocrine System • Pituitary Gland • Thyroid Gland • Parathyroid Glands • Adrenal Glands • Pancreas • Pineal Gland and Thymus • Endocrine Functions of the Kidneys, Heart, Gastrointestinal Tract, and Gonads • Aging and the Endocrine System • Development of the Endocrine System Introduction • ______ glands are ductless organs. • They secrete their molecular products (hormones) into the bloodstream. • All endocrine organs have an extensive distribution of many blood vessels. • The endocrine system and the nervous system both function to communicate signals throughout the body to bring about homeostasis. – Table 20.1 lists similarities and differences between the two organ systems. Comparison of the Endocrine and Nervous Systems Organs of the Endocrine System Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Hypothalamus Antidiuretic hormone (ADH) Oxytocin (OT) Regulatory hormones Pineal gland Pituitary gland Melatonin Anterior pituitary secretes: Adrenocorticotropic hormone (ACTH) Follicle-stimulating hormone (FSH) Growth hormone (GH) Luteinizing hormone (LH) Thyroid gland Parathyroid glands Melanocyte-stimulating hormone (MSH) Calcitonin (CT) (located on posterior surface Prolactin (PRL) Thyroid hormone (TH) of thyroid) Thyroid-stimulating hormone (TSH) Parathyroid hormone (PTH) Posterior pituitary releases: Antidiuretic hormone (ADH) Thymus Oxytocin (OT) Thymopoietin Thymosins Heart Atriopeptin Adrenal glands Gastrointestinal -

The Endodermal Origin of Digestive and Respiratory Tract APUD Cells Histopathologic Evidence and a Review of the Literature Gurdip S
The Endodermal Origin of Digestive and Respiratory Tract APUD Cells Histopathologic Evidence and a Review of the Literature Gurdip S. Sidhu, MD Twenty-seven small cell carcinomas of the lung and three tumors of the large intestine with combined adenocarcinomatous and small cell and/or anaplastic carcinoid-type histologic features were studied by light and electron microscopy. It was shown that the small cells have morphologic characteristics of APUD cells. Also presented are the histologic features of a carcinoma of the lung with large cell undifferentiated carci- noma, adenocarcinoma, squamous cell carcinoma, and giant cell carcinoma areas in the primary site and in several metastatic foci. Two of the renal metastases showed small cell carcinoma. The combined tumors and the numerous other similar neoplasms described in the literature and reviewed here suggest an endodermal origin for diges- tive and respiratory tract APUD cells based on the hypothesis that cancer is a clonal proliferation, and mucous and squamous cell differentiation is an endodermal rather than neural crest characteristic. The ultrastructural features of tumors of cells of known neural crest origin, including a medullary carcinoma of the thyroid, three carotid body tumors, a pheochromocytoma, and two cutaneous melanomas were compared with those of other APUD cell tumors including small cell carcinomas of the lung, two bronchial carcinoids, a carcinoid of the appendix, and a carcinoid of the kidney. Cells of the latter group sometimes possessed cytoplasmic tonofibrils, round compact masses of cytoplasmic microfilaments, and ductal lumina. These features were lacking in the former group and may signify a different embryologic origin. The histologic, histo- pathologic, and embryologic evidence regarding the origin of digestive and respiratory tract APUD cells is reviewed, showing that the former are, and the latter probably are, of endodermal and not neuroectodermal origin. -

Endocrine System 3: Thyroid and Parathyroid Glands
Copyright EMAP Publishing 2021 This article is not for distribution except for journal club use Clinical Practice Keywords Thyroid/Parathyroid/ Endocrine system/Hormones Systems of life This article has been Endocrine system double-blind peer reviewed In this article... ● How thyroid hormones T3 and T4 act to regulate cellular metabolism ● Clinical features of hypothyroidism and hyperthyroidism ● The role of the thyroid and parathyroid glands in maintaining calcium homeostasis Endocrine system 3: thyroid and parathyroid glands Key points Authors John Knight is associate professor in biomedical science; Maria Andrade is The thyroid is in the honorary associate professor in biomedical science; Zubeyde Bayram-Weston is neck, just below the senior lecturer in biomedical science; all at College of Human and Health Sciences, Adam’s apple, and Swansea University. contains four tiny, independently Abstract The endocrine system comprises glands and tissues that produce hormones functioning, to regulate and coordinate vital bodily functions. This article, the third in an eight-part parathyroid glands series on the endocrine system, examines the anatomy and physiology of the thyroid and parathyroid glands, and the pathophysiology associated with some common Thyroid follicular thyroid diseases. cells secrete iodine-rich Citation Knight J et al (2021) Endocrine system 3: thyroid and parathyroid glands. hormones, T3 and Nursing Times [online]; 117: 7, 46-50. T4, which help regulate metabolism his eight-part series on the endo- the adult thyroid gland varies between 15g Release of T3 and crine system opened with an and 30g, and each of the major lobes is T4 is modulated by overview of endocrine glands and around 4cm long and 2cm wide (Benvenga hypothalamus and Tthe role of hormones as chemical et al, 2018; Dorion, 2017). -

Investigations on Congenital and Induced Osteopetrosis
Investigations on Congenital and Induced Osteopetrosis DONALD G. WALKER Department of Anatomy, The Johns Hopkins University School of Medicine, Baltimore, Maryland 21205 Osteopetrosis, or marble bone roid body and bone obtained from thermore, the fact that bone ero disease, is a disturbance of skeletal a normal littermate, little or no evi sion failed to occur, even when the development in which the rate of dence of bone erosion was demon parathyroid body was in direct con bone resorption fails to keep pace strable at the end of the culture tact with the calvarial bone, rules with the rate of bone formation. period. out the possibility that a parathor Bone matrix accumulates exces In another experiment (Barnicot, mone-destroying tissue intervenes sively throughout the skeletal sys 1941) , ribs from a grey-lethal between the gland and its target. tem, causing damage to neighbor mouse were transplanted into a In seeking additional information ing tissues-particularly the dental, normal littermate. When exam as a basis for an alternative to hematopoietic and nervous tissues. ined histologically two weeks later, Barnicot's explanation, I first de The line of investigation on the rib grafts appeared normal. voted my efforts to measuring the osteopetrosis which has led to the In the reciprocal transplantation, plasma calcium concentration in current point of view that the thy in which a rib from a normal untreated animals of both sexes at roid gland represents the primary mouse was cultured subcutaneously various ages. All of the grey-lethals site of the disturbance was initiated in a grey-lethal littermate, the graft examined, including the younger soon after an experimental animal underwent osteopetrotic changes.