The Endodermal Origin of Digestive and Respiratory Tract APUD Cells Histopathologic Evidence and a Review of the Literature Gurdip S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cholecystokinin Expression in the Developing and Regenerating Pancreas and Intestine
233 Cholecystokinin expression in the developing and regenerating pancreas and intestine G Liu, S V Pakala, D Gu, T Krahl, L Mocnik and N Sarvetnick Department of Immunology, Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA (Requests for offprints should be addressed to N Sarvetnick; Email: [email protected]) Abstract In developmental terms, the endocrine system of neither NOD mice continued this pattern. By contrast, in IFN- the gut nor the pancreatic islets has been characterized transgenic mice, CCK expression was suppressed from fully. Little is known about the involvement of cholecysto- birth to 3 months of age in the pancreata but not intestines. kinin (CCK), a gut hormone, involved in regulating the However, by 5 months of age, CCK expression appeared secretion of pancreatic hormones, and pancreatic growth. in the regenerating pancreatic ductal region of IFN- Here, we tracked CCK-expressing cells in the intestines transgenic mice. In the intestine, CCK expression per- and pancreata of normal mice (BALB/c), Non Obese sisted from fetus to adulthood and was not influenced Diabetic (NOD) mice and interferon (IFN)- transgenic by IFN-. Intestinal cells expressing CCK did not mice, which exhibit pancreatic regeneration, during em- co-express glucagon, suggesting that these cells are bryonic development, the postnatal period and adulthood. phenotypically distinct from CCK-expressing cells in We also questioned whether IFN- influences the expres- the pancreatic islets, and the effect of IFN- on sion of CCK. The results from embryonic day 16 showed CCK varies depending upon the cytokine’s specific that all three strains had CCK in the acinar region of microenvironment. -

Endocrine Pancreatic Tumors: Ultrastructure
ANNALS OF CLINICAL AND LABORATORY SCIENCE, Vol. 10, No. 1 Copyright© 1980, Institute for Clinical Science, Inc. Endocrine Pancreatic Tumors: Ultrastructure MERY KOSTIANOVSKY, M.D. Department of Pathology, Thomas Jefferson University, Philadelphia, PA 19107 ABSTRACT Endocrine pancreatic tumors are frequently multicellular and produce several hormones and peptides. A review of the basic concepts of hormone secretion, pancreatic islet cell composition and ultrastructural make-up of tumors is presented. The importance of correlating ultrastructural, immuno- cytochemical and biochemical studies of these tumors is emphasized. Introduction Morphofunctional Aspects of Pancreatic Islets During the last few years a great amount of information was accumulated regarding At the present time four different types the mechanisms of synthesis, storage and of cells have been described in the pan release of hormones.14,28,31 The use of ex creatic islets,17,33 each having a specific perimental in vitro m odels21,25,26,28 was secretory product (table I). A variety of very helpful in clarifying the participation other cells possibly exists, although of different organelles in the biosynthesis, further identification is awaited. By light cellular “packaging” and emyocytosis of microscopy, it is not possible to distin the secretory products. In a review, Lacy31 guish one type of cell from the other. has proposed a working model for hor Histochemical procedures are of help, mone secretion, describing the sim however, and B cells are easily stained ilarities between different endocrine with aldehyde fuchsin. The dicferent pro glands. Some of this information was ob cedures and empiric nature oi the silver tained through the studies of endocrine stain22 added confusion in the nomen pancreatic tumors as in the case of the clature of the cells (as seen in table I) discovery of pro-insulin in a beta cell where the same cell has been described adenoma.62 The purpose of this paper is to by different names. -

Reference ID: 4125998
HIGHLIGHTS OF PRESCRIBING INFORMATION • Determine the number of vials to be reconstituted based on the patient’s These highlights do not include all the information needed to use weight and prescribed dose (2.2) ® CHIRHOSTIM safely and effectively. See full prescribing information for • ChiRhoStim® must be reconstituted with 0.9% Sodium Chloride CHIRHOSTIM®. Injection prior to administration (2.2) • See full prescribing information for complete information on exocrine ® CHIRHOSTIM (human secretin) for injection, for intravenous use test methods (2.3) Initial U.S. Approval: 2004 -------------------------RECENT MAJOR CHANGES---------------------------- ---------------------DOSAGE FORMS AND STRENGTHS--------------------- Dosage and Administration (2.1) 07/2017 For injection: 16 mcg or 40 mcg of human secretin as a lyophilized powder in Contraindications, removed (4) 07/2017 single-dose vial for reconstitution (3) Warnings and Precautions (5.1, 5.2) 07/2017 -------------------------------CONTRAINDICATIONS----------------------------- -------------------------INDICATIONS AND USAGE----------------------------- None (4) ChiRhoStim® is a secretin class hormone indicated for stimulation of: • pancreatic secretions, including bicarbonate, to aid in the diagnosis of -----------------------WARNINGS AND PRECAUTIONS----------------------- exocrine pancreas dysfunction (1) • Hyporesponse to Secretin Stimulation Testing in Patients with • gastrin secretion to aid in the diagnosis of gastrinoma (1) Vagotomy, Inflammatory Bowel Disease or Receiving -

Digestive System Physiology of the Pancreas
Digestive System Physiology of the pancreas Dr. Hana Alzamil Objectives Pancreatic acini Pancreatic secretion Pancreatic enzymes Control of pancreatic secretion ◦ Neural ◦ Hormonal Secretin Cholecystokinin What are the types of glands? Anatomy of pancreas Objectives Pancreatic acini Pancreatic secretion Pancreatic enzymes Control of pancreatic secretion ◦ Neural ◦ Hormonal Secretin Cholecystokinin Histology of the Pancreas Acini ◦ Exocrine ◦ 99% of gland Islets of Langerhans ◦ Endocrine ◦ 1% of gland Secretory function of pancreas Acinar and ductal cells in the exocrine pancreas form a close functional unit. Pancreatic acini secrete the pancreatic digestive enzymes. The ductal cells secrete large volumes of sodium bicarbonate solution The combined product of enzymes and sodium bicarbonate solution then flows through a long pancreatic duct Pancreatic duct joins the common hepatic duct to form hepatopancreatic ampulla The ampulla empties its content through papilla of vater which is surrounded by sphincter of oddi Objectives Pancreatic acini Pancreatic secretion Pancreatic enzymes Control of pancreatic secretion ◦ Neural ◦ Hormonal Secretin Cholecystokinin Composition of Pancreatic Juice Contains ◦ Water ◦ Sodium bicarbonate ◦ Digestive enzymes Pancreatic amylase pancreatic lipase Pancreatic nucleases Pancreatic proteases Functions of pancreatic secretion Fluid (pH from 7.6 to 9.0) ◦ acts as a vehicle to carry inactive proteolytic enzymes to the duodenal lumen ◦ Neutralizes acidic gastric secretion Enzymes ◦ -

Histogenesis of Medullary Carcinoma of the Thyroid
J. clin. Path., (1966), 19, 114 Histogenesis of medullary carcinoma of the thyroid E. D. WILLIAMS1 From the Institute ofPathology, The London Hospital SYNOPSIS Thirty-one dog thyroid tumours and 28 spontaneous rat thyroid tumours were studied histologically and the findings compared with those of a study of 67 cases of medullary carcinoma of the human thyroid. Five of the dog tumours and 24 of the rat tumours were considered to belong to the same group of tumours as medullary carcinoma, a group characterized by solid sheets or lobules of uniform cells with granular cytoplasm and without papillary or follicular differentiation. In the rat tumours it was shown that the cell of origin was the parafollicular cell and not the thyroid follicle epithelial cell. It is suggested that medullary carcinoma is also derived from a parafollicular cell and that this origin would resolve the discrepancy between the relatively good prognosis and the apparently undifferentiated structure of this tumour. It is also concluded that the whole spectrum of clinical and pathological features of medullary carcinoma makes more sense if it is considered as a parafollicular cell tumour. Medullary carcinoma of the thyroid is a distinct the other 19 tumours, two were classified as papillary and clearly defined entity, and the histological carcinomas, nine as follicular carcinomas, two as features of 67 examples are discussed in the previous anaplastic carcinomas, while one tumour showed a paper (Williams, Brown, and Doniach, 1966). The mixture of follicular carcinoma with osteochondro- survival of patients with this tumour is often pro- sarcoma. The remaining five tumours were similar longed although paradoxically the vast majority of and were made up of nests ofuniform cells, separated cases show no evidence of differentiation towards a into groups by a connective tissue stroma which was thyroid epithelial pattern. -

Normal Pancreatic Function 1. What Are the Functions of the Pancreas?
Normal Pancreatic Function Stephen J. Pandol Cedars-Sinai Medical Center and Department of Veterans Affairs Los Angeles, California USA [email protected] Version 1.0, June 13, 2015 [DOI: 10.3998/panc.2015.17] 1. What are the functions of the This chapter presents processes underlying the functions of the exocrine pancreas with pancreas? references to how specific abnormalities of the The pancreas has both exocrine and endocrine pancreas can lead to disease states. function. This chapter is devoted to the exocrine functions of the pancreas. The exocrine function 2. Where is the pancreas located? is devoted to secretion of digestive enzymes, ions and water into the intestine of the gastrointestinal The illustration in Figure 1 demonstrates the (GI) tract. The digestive enzymes are necessary anatomical relationships between the pancreas for converting a meal into molecules that can be and organs surrounding it in the abdomen. The absorbed across the surface lining of the GI tract regions of the pancreas are the head, body, tail into the body. Of note, there are digestive and uncinate process (Figure 2). The distal end enzymes secreted by our salivary glands, of the common bile duct passes through the head stomach and surface epithelium of the GI tract of the pancreas and joins the pancreatic duct as it that also contribute to digestion of a meal. enters the intestine (Figure 2). Because the bile However, the exocrine pancreas is necessary for duct passes through the pancreas before entering most of the digestion of a meal and without it the intestine, diseases of the pancreas such as a there is a substantial loss of digestion that results cancer at the head of the pancreas or swelling in malnutrition. -

Endocrine Glands
Endocrine glands David Kachlík Endocrine system • one out if two regulator systems • phylogenetically older than the nervous system • regulates activity of other systems so that they could react to changing requirements of outer and inner environment (maintains homeostasis) • does not originate from anatomically similar structures • secretion into blood – possesses no ducts • nearly all organs and tissues of the human body produce a hormone Hormone • horman in Greek = to arise • chemical messanger produced by endocrine gland and transported into blood to target organs • proteins (polypeptides) – insuline • amines – adrenaline • steroids – estrogenes Clinical consequence • hormonal excess – primary gland overproduction – secondary to excess production of trophic (releasing, stimulating) substance (hormone) • hormonal deficiency – primary gland failure – secondary to lack of stimulation by trophic (releasing, stimulating) substance (hormone) – target organ resistance Endocrine glands History Thomas Wharton • 1614-1673 • Adenographia • first detailed description of glands Ernest Henry Starling • 1866-1927 • general schemes of „endocrine secretion“ • used the already exsiting word „hormones“ Endocrine system arrangement • glands • disseminated cells • neuroendocrine cells Endocrine glands – list • hypothalamus (hypothalamus) • pituitary gland (hypophysis; gl. pituitaria) • thyroid gland (glandula thyroidea) • parathyroid bodies (gll. parathyroideae) • suprarenal glands, adrenals (gll. suprarenales) • pancreatic (Langerhans‘) island (insulae -

A Case of Calcitonin Producing Adenoma of the Thyroid
Endocrinol. Japon. 1984, 31 (1), 63-70 A Case of Calcitonin Producing Adenoma of the Thyroid TAKAYA KODAMA, MASAKO TAMURA, YOSHIHARU KANAJI, YUKIO ITO, TAKAO OBARA, YOSHIHIDE FUJIMOTO AND AKIRA HIRAYAMA* Department of Endocrine Surgery and Department of Surgical Pathology*, Tokyo Women's Medical College. Ichigaya-Kawadacho, Shinjuku-ku, Tokyo 162 Abstract A thyroid tumor about 4 cm in diameter was removed from a 53-year-old female. The clinical features were those of common thyroid adenomas, such as soft, smooth-surfaced and round contour of the tumor, no calcification and no lymph node metastasis. However, microscopically, the tumor was composed of compact cell nests rather similar to paraganglioma without any follicle formation. Histochemical examination revealed its C-cell nature, such as argyrophilia and calcitonin activity, although no amyloid deposit was demonstrated. Extremely high levels of calcitonin were found in the stored serum taken pre-operatively, but serum carcinoembryonic antigen levels were within normal limits. The calcitonin level dropped to the normal range soon after the operation, indicating the absence of residual tumorous tissues. Thus, the tumor behaves as an adenoma with a C-cell nature. Interestingly enough, the benign counterpart of medullary carcinoma of the thyroid seems to be quite rare in humans, and no similar tumors have ever been reported. Variations of C-cell tumors will be discussed in relation to their production and secretion of carcinoembryonic antigen. Medullary carcinoma of the thyroid hormones, confirming the C-cell as the origin (MCT) was first regarded as a clinical entity of MCT. by Hazard et al. (1959), recognizing a group A wide variation of morphological futures of solid non-follicular tumors with com- and prognosis of MCT had already been paratively good prognosis among apparently pointed out by Williams et al. -
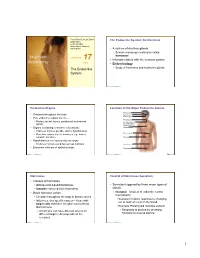
The Endocrine System
PowerPoint® Lecture Slides The Endocrine System: An Overview prepared by Leslie Hendon University of Alabama, Birmingham • A system of ductless glands • Secrete messenger molecules called hormones C H A P T E R 17 • Interacts closely with the nervous system Part 1 • Endocrinology The Endocrine • Study of hormones and endocrine glands System Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Endocrine Organs Location of the Major Endocrine Glands Pineal gland • Scattered throughout the body Hypothalamus Pituitary gland • Pure endocrine organs are the … Thyroid gland • Pituitary, pineal, thyroid, parathyroid, and adrenal Parathyroid glands glands (on dorsal aspect of thyroid gland) • Organs containing endocrine cells include: Thymus • Pancreas, thymus, gonads, and the hypothalamus Adrenal glands • Plus other organs secrete hormones (eg., kidney, stomach, intestine) Pancreas • Hypothalamus is a neuroendocrine organ • Produces hormones and has nervous functions Ovary (female) Endocrine cells are of epithelial origin • Testis (male) Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Figure 17.1 Hormones Control of Hormones Secretion • Classes of hormones • Amino acid–based hormones • Secretion triggered by three major types of • Steroids—derived from cholesterol stimuli: • Basic hormone action • Humoral—simplest of endocrine control mechanisms • Circulate throughout the body in blood vessels • Secretion in direct response to changing • Influences only specific tissues— those with ion or nutrient levels in the blood target cells that have receptor molecules for that hormone • Example: Parathyroid monitors calcium • A hormone can have different effects on • Responds to decline by secreting different target cells (depends on the hormone to reverse decline receptor) Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. -

Physiology of the Pancreas
Te xt . Only in Females’ slide . Only in Males’ slides . Important Lecture . Numbers No.4 . Doctor notes . Notes and explanation “Be The Best Version Of You” 1 We recommended you to study Histology & Anatomy of pancreas first. Physiology of the pancreas Objectives: 1-Functional Anatomy. 2-Major components of pancreatic juice and their physiologic roles. 3-Cellular mechanisms of bicarbonate secretion. 4-Cellular mechanisms of enzyme secretion. 5-Activation of pancreatic enzymes. 6-Hormonal & neural regulation of pancreatic secretion. 7-Potentiation of the secretory response. 8- Pancreatic acini. 2 Functional Anatomy & Histology of the Pancreas Today we are concern about the digestive role of pancreas not the endocrine role, the endocrine part represent 95% of its Extra function, the left 5% represent the digestive part. Functional Anatomy: The pancreas, which lies parallel to and beneath the stomach is a large compound gland with most of its internal structure similar to that of the salivary glands (Look like them so they have both acinar cells and tubal cells) Islet of langerhans in the pancreas: the pancreas, in addition to its digestive functions, secretes two important hormones: Insulin (beta cells, 60%). Islet of Langerhans have different type of cells (alpha, beta and delta) and If we go and get a cross section view of the every single one of them can release a certain hormone. pancreatic tissue. We can see many acinar Glucagon (alpha cells, ~25%). cells and in the middle we have a ductal That are crucial for normal regulation of glucose, lipid, and protein metabolism. Also somatostatin is lumen. secreted by delta cells (form ~10% of islets’s cells). -

Anatomy of Endocrine System
Anatomy of Endocrine system Introduction, Pituitary gland and Thyroid gland Prepared by Dr. Payal Jain Endocrine System I. Introduction A. Considered to be part of animals communication system 1. Nervous system uses physical structures for communication 2. Endocrine system uses body fluids to transport messages (hormones) II. Hormones A. Classically, hormones are defined as chemical substances produced by ductless glands and secreted into the blood supply to affect a tissue distant from the gland, but now it is understood that hormones can be produced by single cells as well. 1. epicrine a. hormones pass through gap junctions of adjacent cells without entering extracellular fluid 2. paracrine a. hormones diffuse through interstitial fluid (e.g. prostaglandins) 3. endocrine a. hormones are delivered via the bloodstream (e.g. growth hormone Different endocrine glands with cell Organ Division arrangement Cell arrangement/morphology Hormone Hypophysis Adenohypophysis Pars distalis Cells in cords around large-bore capillaries: Acidophils Growth hormone, prolactin Basophils ACTH, TSH, FSH, LH Pars intermedia Mostly basophilic cells around ACTH, POMC cystic cavities Pars tuberalis Narrow sleeve of basophilc cells LH around infundibulum Neurohypophysis Pars nervosa Nerve fibers and supporting cells Oxytocin and (pituicytes) vasopressin (produced in hypothalamus) Infundibulum Nerve fibers (traveling from hypothalamus to pars nervosa) Pancreas Islet of Langerhans Irregularly arranged cells with Insulin, glucagon many capillaries Follicles: Simple -

Duodenal Acidity May Increase the Risk of Pancreatic Cancer in the Course of Chronic Pancreatitis: an Etiopathogenetic Hypothesis
JOP. J Pancreas (Online) 2005; 6(2):122-127. EDITORIAL Duodenal Acidity May Increase the Risk of Pancreatic Cancer in the Course of Chronic Pancreatitis: An Etiopathogenetic Hypothesis Giorgio Talamini Gastroenterology and Endoscopy Service, University of Verona. Verona, Italy Summary duodenum. Patients undergoing a Whipple procedure or side-to-side pancreaticojejuno- Chronic pancreatitis patients have an stomy are probably less critically affected increased risk of developing pancreatic because secretions transit, at least in part, via cancer. The cause of this increase has yet to the papilla. be fully explained but smoking and If the duodenal acidity hypothesis proves inflammation may play an important role. To correct, then, in addition to stopping smoking, these, we must now add a new potential risk reduction of duodenal acid load in patients factor, namely duodenal acidity. Patients with with pancreatic insufficiency may help chronic pancreatitis very often present decrease the risk of pancreatic cancer. pancreatic exocrine insufficiency combined with a persistently low duodenal pH in the postprandial period. The duodenal mucosa in Chronic pancreatitis (CP) patients have an chronic pancreas patients with pancreatic increased risk of developing pancreatic cancer insufficiency has a normal concentration of s- [1, 2]. The cause of this increase has yet to be cells and, therefore, the production of secretin fully explained and various hypotheses are is preserved. Pancreatic ductal cells are being explored [3, 4, 5, 6, 7]. Nevertheless, largely responsible for the amount of smoking unquestionably plays an important bicarbonate and water secretion in response to role [4, 8, 9] because the great majority of CP secretin stimulation.