Gymnopithys Lunulata) En Ecuador
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Perú: Cordillera Escalera-Loreto Perú: Cordillera Escalera-Loreto Escalera-Loreto Cordillera Perú: Instituciones Participantes/ Participating Institutions
.................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................... .............................................................................................................................................................................................................................................................................................................................................................................................no. 26 ....................................................................................................................... 26 Perú: Cordillera Escalera-Loreto Perú: Cordillera Escalera-Loreto Instituciones participantes/ Participating Institutions The Field Museum Nature and Culture International (NCI) Federación de Comunidades Nativas Chayahuita (FECONACHA) Organización Shawi del Yanayacu y Alto Paranapura (OSHAYAAP) Municipalidad Distrital de Balsapuerto Instituto de Investigaciones de la Amazonía Peruana (IIAP) Herbario Amazonense de la Universidad Nacional de la Amazonía Peruana (AMAZ) Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos Centro -

The Behavior of Plain-Brown Woodcreepers, Dendrocincla Fuliginosa
THE BEHAVIOR OF PLAIN-BROWN WOODCREEPERS, DENDROCINCLA FULIGINOSA EDWIN 0. WILLIS N forests of tropical America, woodcreepers of the genus Dendrocincla I follow swarms of army ants persistently (Willis, 1960:158-159; Skutch, 1969:136; Oniki and Willis, 1972). Intensive studies of ant-following birds on Barro Colorado Island, Panama Canal Zone, and brief studies in other areas show that Plain-brown Woodcreepers regularly follow army ants. The changes in foraging niche when these woodcreepers confront different sets of competing antbirds at some of the localities have been detailed elsewhere (Willis, 1966). Here social and individual behavior will be considered. Feduccia (197O:I) lists many brief references, mostly in annotated lists, on the behavior of woodcreepers. The only extensive studies have been Skutchs’ (1969) of Tawny-winged and other woodcreepers. Slud (1960, 1964) and several others, including Johnson (1954) and Snow and Snow (1964) and Oniki (1970) among references not listed by Feduccia, have commented briefly on the natural history of Plain-brown Woodcreepers. The species and its genus and family are not well known ethologically. Appendix 1 lists common and scientific names of birds mentioned herein, following Meyer de Schauensee (1970)) except for Central American birds listed only in Eisenmann (1955) and for northern birds listed in the A.O.U. Check-list. THE PLAIN-BROWN WOODCREEPERS Plain-brown Woodcreepers wait on or hitch up the trunks of trees like slender woodpeckers or overgrown Brown Creepers. They live in the middle and lower levels of humid lowland forests from Honduras to central Brasil. Occasionally they wander to the edge of the forest, into cacao and coffee orchards, or into second growth more than 5 m tall. -

Ecuador Trip Report Andes to the Amazon 5Th to 19Th September 2011 (15 Days)
Ecuador Trip Report Andes to the Amazon 5th to 19th September 2011 (15 days) Giant Antpitta by Luis Segura Tour Leader: Forrest Rowland Some of our tour highlights included: 1. ZigZag Heron 2. Giant Antpitta 3. Dark-backed Wood Quail 4. Lunulated Antbird 5. Toucan Barbet 6. Plate-billed Mountain Toucan 7. Rusty-belted Tapaculo 8. Orange-breasted Fruiteater 9. Long-tailed Potoo 10. Collared Puffbird RBT Ecuador Trip Report 2011 2 Tour Intro Ecuador is quite simply incredible! In the past 15 years, this humble country has become the most traversed and well-known of all South American nations by birders, and for good reason. Despite being no larger in size than the state of Colorado (USA), and occupying less than one-quarter the size of the Republic of Colombia, Ecuador has 30% more bird species than the whole of North America and only 16% less than Colombia. This is no doubt due to the fact that Ecuador’s political boundaries include some of the highest peaks in the Andean chain, perpetually snow-bound, and, of course, the humid rainforests of the Amazon, while two distinct Andean chains result in a dry Inter- Andean Valley with separate rainshadows on the outer slopes, and every habitat that such a varied and rich equatorial topography could possible harbor. The goal of this tour was to provide an introduction to these incredible birding zones. In most countries, 14 days would fall far short in terms of adequately representing such diversity: distances would be too great, travel times prohibitive and, quite often, infrastructure in rural South America just doesn’t allow for any visitors. -
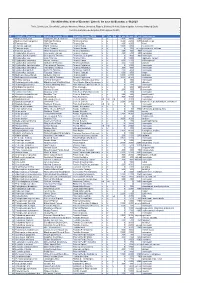
Ecuador Simple List Version 2020 Clements
Checklist of the birds of Ecuador / Lista de las aves del Ecuador, v. 08.2020 Freile, Brinkhuizen, Greenfield, Lysinger, Navarrete, Nilsson, Olmstead, Ridgely, Sánchez-Nivicela, Solano-Ugalde, Athanas, Ahlman & Boyla Comité Ecuatoriano de Registros Ornitológicos (CERO) ID Scientific_Clements_2019 English_Clements_2019 Español Ecuador CERO EC Con Gal Alt_min Alt_max Alt_ext Subespecies 1 Nothocercus julius Tawny-breasted Tinamou Tinamú Pechileonado x x 2300 3400 2100 monotypic 2 Nothocercus bonapartei Highland Tinamou Tinamú Serrano x x 1600 2200 3075 plumbeiceps 3 Tinamus tao Gray Tinamou Tinamú Gris x x 400 1600 kleei 4 Tinamus osgoodi Black Tinamou Tinamú Negro x x 1000 1400 hershkovitzi 5 Tinamus major Great Tinamou Tinamú Grande x x 0 700 1200, 1350 peruvianus, latifrons 6 Tinamus guttatus White-throated Tinamou Tinamú Goliblanco x x 200 400 900 monotypic 7 Crypturellus cinereus Cinereous Tinamou Tinamú Cinéreo x x 200 600 900 monotypic 8 Crypturellus berlepschi Berlepsch's Tinamou Tinamú de Berlepsch x x 0 400 900 monotypic 9 Crypturellus soui Little Tinamou Tinamú Chico x x 0 1200 nigriceps, harterti 10 Crypturellus obsoletus Brown Tinamou Tinamú Pardo x x 500 1100 chirimotanus? 11 Crypturellus undulatus Undulated Tinamou Tinamú Ondulado x x 200 600 yapura 12 Crypturellus transfasciatus Pale-browed Tinamou Tinamú Cejiblanco x x 0 1600 monotypic 13 Crypturellus variegatus Variegated Tinamou Tinamú Abigarrado x x 200 400 monotypic 14 Crypturellus bartletti Bartlett's Tinamou Tinamú de Bartlett x x 200 400 monotypic 15 Crypturellus -

Phlegopsis Erythroptera (Gould, 1855) and Relatives (Aves, Formicariidae) As Army Ant Followers
REVISTA BRASILEIRA DE ZOOLOGIA Revta bras. Zoo1., S P aulo 2 (3): 165-170 31. V .1984 PHLEGOPSIS ERYTHROPTERA (GOULD, 1855) AND RELATIVES (AVES, FORMICARIIDAE) AS ARMY ANT FOLLOWERS EDWIN O. WILLIS ABSTRACT Phlegopsis erythroptera (Formicariidae) follows army ants regularly for flushed arthropods between the Andes and the Negro/Madeira Rivers. Mainly a bird of terra firme forests, it is interspecifically aggressive. Low numbers at ant swarms are probably due to lOlO productivity of arthropods flushed by ants on weathered terra firme soils, or to high species diversity of subordinate but active ant-following competitors in upper Amazonia. Sexual dimorphism of young and female erythroptera is attributed to low numbers over ants, so that dispersed individuals avoíd attacks by bright-plumaged adult males rather than bluft them out at close range. Phlegopsis, Skutchia, Rhegmatorhina, and Gymnopithys are related to and perhaps congeneric with Pithys; all follow ants and se em a group derived from birds related to Hylophylax. Antbirds of Phlegopsis and related genera (Formicariidae) regularly capture arthropods flushed by swarms Df army ants in neotropical forests (Willis & Oniki, 1978; Willis, 1979). Here, 'in the twentieth report of a series on occasionally-observed ant followers, I note new information on Phlegopsis erythropte'ra and related species. RESULTS 1. Phlegopsis erythroptera (Reddish-winged Bare-Eye), a bird never seen away from ants, followed 64 ant raids in Colombia (7 Umbria, 10 Mitú, 3 Leticia), Ecuador (7 Limoncocha, 3 Zatzayacu 900 m, 10 Yaapi, 9 Putuimi), Peru (5 Andoas), and western Brazil (1 Benjamin Constant, 4 Carauari, 3 Manacapuru, 2 Igapó-Açu). -

Phylogenetic and Biogeographic Relationships in the Neotropical Genus Gymnopithys (Formicariidae)
Wilson Bull., 105(2), 1993, pp. 301-315 PHYLOGENETIC AND BIOGEOGRAPHIC RELATIONSHIPS IN THE NEOTROPICAL GENUS GYMNOPITHYS (FORMICARIIDAE) SHANNON J. HACKIX+* AL%sTRACT.- Evolutionary relationships among obligate ant-following birds in the genus Gymnopithyswere addressed using phenetic and phylogenetic analyses of allozyme char- acters. Genetic variation at 37 gene loci was analyzed across all four species in the genus and within two species (Bicolored Antbird [G. leucaspis],and White-throated Antbird [G. salvin$. Interspecific genetic distances were high, and comparable to other studies of Neo- tropical birds, which exceed those in many temperate zone species. Within the genus, Lunulated Antbird (G. lunulata) and G. salviniwere sister taxa. There was only weak support for a sister-taxon relationship between G. leucaspisand the Rufous-throated Antbird (G. n&da). Within G. leucaspisand G. salvini, high F,, indicated substantial genetic subdi- vision, again comparable to other Neotropical birds and much greater than temperate zone birds. Increased age of population isolation is proposed to account for the high genetic divergence in Neotropical birds. ReceivedI5 May 1992, accepted24 Nov. 1992. Despite widespread interest in biogeographic patterns of Amazonian birds, few phylogenies of Neotropical birds and no analyses of the genetic structure of widespread Amazonian species have been published. In this paper, I address phylogenetic and biogeographic relationships among pop- ulations within two widespread species of Gymnopithys antbirds (For- micariidae) and among all four species in the genus using allozyme char- acters. In addition, I summarize and add to the growing body of genetic information on Neotropical forest birds. All species in the genus Gymnopithys are obligate ant-following birds (Willis 1967, 1968) distributed throughout lowland forests of Central and South America (from Honduras south to Brazil). -

Eastern Ecuador: February-March 2020
Tropical Birding - Trip Report Eastern Ecuador: February-March 2020 A Tropical Birding SET DEPARTURE tour Eastern Ecuador: High Andes to Vast Amazon nd th 22 February- 7 March 2020 Hoatzin at Napo Wildlife Center TOUR LEADER: José Illanes Trip report and photos by José Illanes INTRODUCTION: Ecuador is one of the most diverse countries in the World for birds and this trip provided plentiful evidence of this; just over 600 species were recorded on this trip. The reason for such totals was that we birded from high up, above the treeline in the Andes, all the way down the lowland tropical forests of the Amazon, with cloudforests and Andean foothills birded in between these extremes. Early highlights, from the higher elevations of the trip included, Andean Condor, Black-faced Ibis, and Rufous-bellied Seedsnipe. www.tropicalbirding.com +1-409-515-9110 [email protected] p.1 Tropical Birding - Trip Report Eastern Ecuador: February-March 2020 Downhill from there the forests of the subtropics and foothills yielded Military Macaw, Ocellated Tapaculo, Coppery-chested Jacamar, White-bellied and Plain-backed Antpittas, White-capped Tanager, and a wonderful set of hummingbirds, including Sword-billed Hummingbird, Napo Sabrewing, and Gould’s Jewelfront. Then there was the Amazon itself, with birds like Great Jacamar, lots of antbirds, including Lunulated Antbird, and Zigzag Heron, Crested Owl, and Plum-throated Cotinga, not to mention plentiful trogons, toucans, and parrots, as well as mammals like Golden-mantled Tamarin, Giant River Otter, and Spix’s Night Monkey. From the bird list, the participants highlighted the following ones among their favorites: Ocellated Tapaculo, Rufous-bellied Seedsnipe, Zigzag Heron, Chestnut-crowned Gnateater, Crested Quetzal, Wire-tailed Manakin, Ochre-breasted Antpitta, Andean Cock-of-the Rock, Sparkling Violetear, Wire-crested Thorntail, Gray-breasted Mountain-Toucan, Scarlet Macaw, Ecuadorian Hillstar and the bizarre Hoatzin. -

New Locality for White-Masked Antbird Pithys Castaneus and Other Avian Range Extensions for Dpto
Cotinga 39 New locality for White-masked Antbird Pithys castaneus and other avian range extensions for dpto. Loreto, Peru Fabrice Schmitt, Raphaël Sané, Marc Thibault and Gabriel Vásquez Received 3 May 2015; final revision accepted 1 October 2016 Cotinga 39 (2017): OL 1–9 published online 2 March 2017 Presentamos los resultados de una expedición realizada en junio del 2013 cerca del distrito de San Lorenzo, depto. Loreto, Perú. El descubrimiento más notable es una nueva localidad para Pithys castaneus y una población de Epinecrophylla sp., la cual presenta características fenotípicas intermedias entre E. fjeldsaai y E. haematonota. Pensamos que esa población de Epinecrophylla apoya la idea que E. fjeldsaai debería considerarse como una subespecie de E. haematonota. También documentamos extensiones de rango de distribución para otras cuatro especies: Cypseloides cryptus, Polytmus theresiae, Conopias trivirgatus y Tachyphonus rufus. Nuestra lista total de 252 especies incluye varias especies de ocurrencia poco común o raras, tales como Anurolimnas fasciatus, Bucco capensis, B. tamatia, Neoctantes niger, Neopipo cinnamomea y Phoenicircus nigricollis. Con estos resultados esperamos promover en las autoridades locales y regionales acciones de conservación en esta área amenazada por deforestación. Few ornithological expeditions have been boundaries of this general area. Álvarez Alonso1 undertaken in the Amazon lowlands north of the made the only bird inventory along the río Morona río Marañón and west of the río Tigre, in Peru. and we are unaware of any similar survey along Brooks et al.3 compiled the results of >60 years the Peruvian stretch of the río Pastaza. Motivated of exploration in the Pongos area, while Pitman by the recent rediscovery of White-masked Antbird et al.7 reported a Rapid Biological Assessment of Pithys castaneus along the Morona5, our team, the Cordillera de Kampankis, both at the western comprising the authors, R. -

Eastern Ecuador Tour November-December 2010
Tropical Birding Eastern Ecuador Tour November-December 2010 Eastern Ecuador Tour : High Andes to Vast Amazon This rare Andean Potoo, here on a nest, was one of the tour highlights. 27 November - 12 December, 2010 Guided by Andrew Spencer www.tropicalbirding.com [email protected] +1-409-515-0514 Tropical Birding Eastern Ecuador Tour November-December 2010 The Amazon. Merely hearing that word conjures up images of vast, unbroken rainforests teeming with life to an extent unequaled anywhere else on the planet. To a birder it means hundreds of bird species of exotic colors, shapes, and behaviors. It is, in short, one of the ultimate wildlife (and birding) experiences in the world, not to be missed, and hopefully experienced time after time. Ecuador has a good share of this natural cornucopia. In fact, the Amazonian forests in Ecuador are among the best in the in the entire basin, and it is not unusual to rack up a large trip list when visiting the area. This tour was no exception, and when combined with the wide variety of habitats from the high páramo of Papallacta Pass through the temperate and subtropical forests of the Andes, we had a highly successful trip. Highlights ranged from the ptarmigan-esque Rufous-bellied Seedsnipe to the rarely seen Greater Scythebill to the glowingly red Black-necked Red-Cotinga . In total we scored just over 600 species on this excellent tour of Ecuador´s east that covered everything from the chilly high Andes right down into the steamy lowland jungles of the Amazon Basin. Day 1: Quito to Guango Somewhat bizarrely, Ecuador was conducting a census on the first day of the tour, and as a result the roads throughout the country were completely off limits to everyone during most of the daylight hours. -

Global Conservation Significance of Ecuador's Yasunı National Park
CORE Metadata, citation and similar papers at core.ac.uk Provided by PubMed Central Global Conservation Significance of Ecuador’s Yasunı´ National Park Margot S. Bass1, Matt Finer2*, Clinton N. Jenkins3,4, Holger Kreft5, Diego F. Cisneros-Heredia6,7, Shawn F. McCracken8,9, Nigel C. A. Pitman3, Peter H. English10, Kelly Swing7, Gorky Villa1, Anthony Di Fiore11, Christian C. Voigt12, Thomas H. Kunz13 1 Finding Species, Takoma Park, Maryland, United States of America, 2 Save America’s Forests, Washington D. C., United States of America, 3 Nicholas School of the Environment, Duke University, Durham, North Carolina, United States of America, 4 Department of Biology, University of Maryland, College Park, Maryland, United States of America, 5 Division of Biological Sciences, University of California San Diego, La Jolla, California, United States of America, 6 Department of Geography, King’s College London, Strand, London, United Kingdom, 7 College of Biological and Environmental Sciences, Universidad San Francisco de Quito, Quito, Ecuador, 8 Department of Biology, Texas State University, San Marcos, Texas, United States of America, 9 TADPOLE Organization, San Marcos, Texas, United States of America, 10 School of Biological Sciences, University of Texas at Austin, Austin, Texas, United States of America, 11 Department of Anthropology, New York University, New York, New York, United States of America, 12 Leibniz Institute for Zoo and Wildlife Research, Berlin, Germany, 13 Center for Ecology and Conservation Biology, Department of Biology, Boston University, Boston, Massachusetts, United States of America Abstract Background: The threats facing Ecuador’s Yasunı´ National Park are emblematic of those confronting the greater western Amazon, one of the world’s last high-biodiversity wilderness areas. -

Peru: White-Masked Antbird Expedition
OK... it’s not an award winner but it is a White-masked Antbird, and it amply demonstrates why this bird is tough to see in the dense dark Am- azonian undergrowth! Although we got some great views through our binoculars, Jono did brilliantly to capture this rare image (Jono Irvine) PERU: WHITE-MASKED ANTBIRD EXPEDITION 1 – 6 OCTOBER 2018 LEADER: PETE MORRIS As part of our series of trips to see the remaining species not yet on the Birdquest Lifelist, we hatched a plan to go and look for the little known White-masked Antbird – a spectacular obligate antswarm follower that is endemic to Northern Peru and is known from just a handful of sightings from a couple of sites. Fortunately, one of these sites is relatively accessible and so it was that the fve of us and Carlos met up at Tarapoto airport where we were greeted with our frst surprise! The airport at Yurimaguas was closed for resurfacing, and only open for fights in the afternoons. So instead of a relaxing transfer and a night before our fight, we high-tailed it to Yurimaguas and made the arrangements for our fights, before a late lunch overlooking the mighty Huallaga 1 BirdQuest Tour Report: Peru: White-masked Antbird Expedition 2018 www.birdquest-tours.com The fights to and from San Lorenzo were short and... interesting! (Pete Morris) River. Here our binoculars were quickly called upon as we notched up a few common species including the re- cently split Large-billed Parrotlet as well as Black Caracara, Yellow-billed Tern, White-winged and White-band- ed Swallows, and a few Chestnut-bellied Seedeaters. -

Neotropical Birding 24
>> BIRDING SITES AMAZONIAN ECUADOR Amazon lodges in Ecuador: an overview Sam Woods, Scott Olmstead and José Illanes Every birder – and pretty much every non-birder with a vague interest in the world around them – has heard of the Amazon. But fathoming how to go birding there may not be immediately obvious. This article makes the case for visiting Ecuador’s great Amazonian lodges. orking as bird-tour leaders in South natural destination for one’s first Amazonian America, we are regularly consulted for birding – first and foremost due to its proximity W advice about how and where to bird the to a major international airport (in this case, in Amazon. The well-publicised biological diversity the capital of Quito). After a very short flight from of this immense region makes it an inevitable the high Andes down to the lowlands, travellers place for world birders to visit at some point can continue onward to a variety of excellent in their lives. Because it encompasses several ‘jungle’ lodges, even arriving in time for afternoon countries, however, many are unsure of where to birding and dinner. Moreover, several lodges – make their first (and sometimes only) visit. Even those outlined in this article – are equipped with allowing for a slight bias (given that two of us live comfortable accommodation and provide excellent in the country), we propose Ecuador as the most bird guides to help you find key species. Always on the Amazonian agenda are birds such as 1 Bare-necked Fruitcrow Gymnoderus foetidus, Sani Lodge, December 2018 (Sam Woods/ Tropical Birding Tours).