Phylogenetic and Biogeographic Relationships in the Neotropical Genus Gymnopithys (Formicariidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biodiversity of the Southern Rupununi Savannah World Wildlife Fund and Global Wildlife Conservation
THIS REPORT HAS BEEN PRODUCED IN GUIANAS COLLABORATION VERZICHT APERWITH: Ç 2016 Biodiversity of the Southern Rupununi Savannah World Wildlife Fund and Global Wildlife Conservation 2016 WWF-Guianas Global Wildlife Conservation Guyana Office PO Box 129 285 Irving Street, Queenstown Austin, TX 78767 USA Georgetown, Guyana [email protected] www.wwfguianas.org [email protected] Text: Juliana Persaud, WWF-Guianas, Guyana Office Concept: Francesca Masoero, WWF-Guianas, Guyana Office Design: Sita Sugrim for Kriti Review: Brian O’Shea, Deirdre Jaferally and Indranee Roopsind Map: Oronde Drakes Front cover photos (left to right): Rupununi Savannah © Zach Montes, Giant Ant Eater © Gerard Perreira, Red Siskin © Meshach Pierre, Jaguar © Evi Paemelaere. Inside cover photo: Gallery Forest © Andrew Snyder. OF BIODIVERSITYTHE SOUTHERN RUPUNUNI SAVANNAH. Guyana-South America. World Wildlife Fund and Global Wildlife Conservation 2016 This booklet has been produced and published thanks to: 1 WWF Biodiversity Assessment Team Expedition Southern Rupununi - Guyana. The Southern Rupununi Biodiversity Survey Team / © WWF - GWC. Biodiversity Assessment Team (BAT) Survey. This programme was created by WWF-Guianas in 2013 to contribute to sound land- use planning by filling biodiversity data gaps in critical areas in the Guianas. As far as possible, it also attempts to understand the local context of biodiversity use and the potential threats in order to recommend holistic conservation strategies. The programme brings together local knowledge experts and international scientists to assess priority areas. With each BAT Survey, species new to science or new country records are being discovered. This booklet acknowledges the findings of a BAT Survey carried out during October-November 2013 in the southern Rupununi savannah, at two locations: Kusad Mountain and Parabara. -

Perú: Cordillera Escalera-Loreto Perú: Cordillera Escalera-Loreto Escalera-Loreto Cordillera Perú: Instituciones Participantes/ Participating Institutions
.................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................... .............................................................................................................................................................................................................................................................................................................................................................................................no. 26 ....................................................................................................................... 26 Perú: Cordillera Escalera-Loreto Perú: Cordillera Escalera-Loreto Instituciones participantes/ Participating Institutions The Field Museum Nature and Culture International (NCI) Federación de Comunidades Nativas Chayahuita (FECONACHA) Organización Shawi del Yanayacu y Alto Paranapura (OSHAYAAP) Municipalidad Distrital de Balsapuerto Instituto de Investigaciones de la Amazonía Peruana (IIAP) Herbario Amazonense de la Universidad Nacional de la Amazonía Peruana (AMAZ) Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos Centro -

Predation on Vertebrates by Neotropical Passerine Birds Leonardo E
Lundiana 6(1):57-66, 2005 © 2005 Instituto de Ciências Biológicas - UFMG ISSN 1676-6180 Predation on vertebrates by Neotropical passerine birds Leonardo E. Lopes1,2, Alexandre M. Fernandes1,3 & Miguel Â. Marini1,4 1 Depto. de Biologia Geral, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, 31270-910, Belo Horizonte, MG, Brazil. 2 Current address: Lab. de Ornitologia, Depto. de Zoologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Av. Antônio Carlos, 6627, Pampulha, 31270-910, Belo Horizonte, MG, Brazil. E-mail: [email protected]. 3 Current address: Coleções Zoológicas, Aves, Instituto Nacional de Pesquisas da Amazônia, Avenida André Araújo, 2936, INPA II, 69083-000, Manaus, AM, Brazil. E-mail: [email protected]. 4 Current address: Lab. de Ornitologia, Depto. de Zoologia, Instituto de Biologia, Universidade de Brasília, 70910-900, Brasília, DF, Brazil. E-mail: [email protected] Abstract We investigated if passerine birds act as important predators of small vertebrates within the Neotropics. We surveyed published studies on bird diets, and information on labels of museum specimens, compiling data on the contents of 5,221 stomachs. Eighteen samples (0.3%) presented evidence of predation on vertebrates. Our bibliographic survey also provided records of 203 passerine species preying upon vertebrates, mainly frogs and lizards. Our data suggest that vertebrate predation by passerines is relatively uncommon in the Neotropics and not characteristic of any family. On the other hand, although rare, the ability to prey on vertebrates seems to be widely distributed among Neotropical passerines, which may respond opportunistically to the stimulus of a potential food item. -

Southern Wing-Banded Antbird, Myrmornis Torquata Myrmornithinae
Thamnophilidae: Antbirds, Species Tree I Northern Wing-banded Antbird, Myrmornis stictoptera ⋆Southern Wing-banded Antbird, Myrmornis torquata ⋆ Myrmornithinae Spot-winged Antshrike, Pygiptila stellaris Russet Antshrike, Thamnistes anabatinus Rufescent Antshrike, Thamnistes rufescens Guianan Rufous-rumped Antwren, Euchrepomis guianensus ⋆Western Rufous-rumped Antwren, Euchrepomis callinota Euchrepomidinae Yellow-rumped Antwren, Euchrepomis sharpei Ash-winged Antwren, Euchrepomis spodioptila Chestnut-shouldered Antwren, Euchrepomis humeralis ⋆Stripe-backed Antbird, Myrmorchilus strigilatus ⋆Dot-winged Antwren, Microrhopias quixensis ⋆Yapacana Antbird, Aprositornis disjuncta ⋆Black-throated Antbird, Myrmophylax atrothorax ⋆Gray-bellied Antbird, Ammonastes pelzelni MICRORHOPIINI ⋆Recurve-billed Bushbird, Neoctantes alixii ⋆Black Bushbird, Neoctantes niger Rondonia Bushbird, Neoctantes atrogularis Checker-throated Stipplethroat, Epinecrophylla fulviventris Western Ornate Stipplethroat, Epinecrophylla ornata Eastern Ornate Stipplethroat, Epinecrophylla hoffmannsi Rufous-tailed Stipplethroat, Epinecrophylla erythrura White-eyed Stipplethroat, Epinecrophylla leucophthalma Brown-bellied Stipplethroat, Epinecrophylla gutturalis Foothill Stipplethroat, Epinecrophylla spodionota Madeira Stipplethroat, Epinecrophylla amazonica Roosevelt Stipplethroat, Epinecrophylla dentei Negro Stipplethroat, Epinecrophylla pyrrhonota Brown-backed Stipplethroat, Epinecrophylla fjeldsaai ⋆Napo Stipplethroat, Epinecrophylla haematonota ⋆Streak-capped Antwren, Terenura -

Gymnopithys Lunulata) En Ecuador
SHORT COMMUNICATIONS ORNITOLOGIA NEOTROPICAL 12: 183–185, 2001 © The Neotropical Ornithological Society EXTENSIÓN ALTITUDINAL EN LA DISTRIBUCIÓN DEL HORMIGUERO LUNULADO (GYMNOPITHYS LUNULATA) EN ECUADOR Juan F. Freile1 Departamento de Biología, Pontificia Universidad Católica del Ecuador, Casilla 17-01-2184, Quito, Ecuador. Altitudinal distribution extension of the Lunulated Antbird (Gymnopithys lunulata) in Ecuador. Key words: Gymnopithys lunulata, Lunulated Antbird, altitudinal extension, Amazonia, Ecuador. El Hormiguero Lunulado (Gymnopithys lunu- 1996, Ridgely et al. 1998). lata; Thamnophilidae) es un insectívoro raro En este reporte se presenta el primer que habita al interior de bosque húmedo pri- registro del Hormiguero Lunulado a una alti- mario y se encuentra principalmente asociado tud superior a 300 m. En Noviembre 1999, a bandadas mixtas de sotobosque siguiendo un macho adulto fue capturado en una red de tropas de hormigas arrieras (Eciton spp. y neblina colocada dentro de un pequeño rema- Labidus praedator). Se lo encuentra frecuente- nente de bosque piemontano secundario coli- mente en zonas de varzea, aunque también ha nado a 950 m de altitud, en el Parque sido registrado en bosques de tierra firme Etnobotánico y Pedagógico Omaere (01° 28’S, (Willis 1968, Ridgely & Tudor 1994, observ. 77° 59’W), Provincia de Pastaza. Las medidas pers.). morfométricas de este individuo [culmen 17.7 El rango de distribución del Hormiguero mm, tarso 27.2 mm, ala 69.9 mm (aplanada), Lunulado está restringido a la Amazonía occi- cola 44.0 mm, peso 26.0 g] son similares a las dental, y se extiende desde el nororiente de de otros individuos revisados, provenientes Ecuador hasta el nororiente de Perú (Ridgely de varias localidades de la Amazonía baja del & Tudor 1994). -

The Behavior of Plain-Brown Woodcreepers, Dendrocincla Fuliginosa
THE BEHAVIOR OF PLAIN-BROWN WOODCREEPERS, DENDROCINCLA FULIGINOSA EDWIN 0. WILLIS N forests of tropical America, woodcreepers of the genus Dendrocincla I follow swarms of army ants persistently (Willis, 1960:158-159; Skutch, 1969:136; Oniki and Willis, 1972). Intensive studies of ant-following birds on Barro Colorado Island, Panama Canal Zone, and brief studies in other areas show that Plain-brown Woodcreepers regularly follow army ants. The changes in foraging niche when these woodcreepers confront different sets of competing antbirds at some of the localities have been detailed elsewhere (Willis, 1966). Here social and individual behavior will be considered. Feduccia (197O:I) lists many brief references, mostly in annotated lists, on the behavior of woodcreepers. The only extensive studies have been Skutchs’ (1969) of Tawny-winged and other woodcreepers. Slud (1960, 1964) and several others, including Johnson (1954) and Snow and Snow (1964) and Oniki (1970) among references not listed by Feduccia, have commented briefly on the natural history of Plain-brown Woodcreepers. The species and its genus and family are not well known ethologically. Appendix 1 lists common and scientific names of birds mentioned herein, following Meyer de Schauensee (1970)) except for Central American birds listed only in Eisenmann (1955) and for northern birds listed in the A.O.U. Check-list. THE PLAIN-BROWN WOODCREEPERS Plain-brown Woodcreepers wait on or hitch up the trunks of trees like slender woodpeckers or overgrown Brown Creepers. They live in the middle and lower levels of humid lowland forests from Honduras to central Brasil. Occasionally they wander to the edge of the forest, into cacao and coffee orchards, or into second growth more than 5 m tall. -

An Initial Estimate of Avian Ark Kinds
Answers Research Journal 6 (2013):409–466. www.answersingenesis.org/arj/v6/avian-ark-kinds.pdf An Initial Estimate of Avian Ark Kinds Jean K. Lightner, Liberty University, 1971 University Blvd, Lynchburg, Virginia, 24515. Abstract Creationists recognize that animals were created according to their kinds, but there has been no comprehensive list of what those kinds are. As part of the Answers in Genesis Ark Encounter project, research was initiated in an attempt to more clearly identify and enumerate vertebrate kinds that were SUHVHQWRQWKH$UN,QWKLVSDSHUXVLQJPHWKRGVSUHYLRXVO\GHVFULEHGSXWDWLYHELUGNLQGVDUHLGHQWLÀHG 'XHWRWKHOLPLWHGLQIRUPDWLRQDYDLODEOHDQGWKHIDFWWKDWDYLDQWD[RQRPLFFODVVLÀFDWLRQVVKLIWWKLVVKRXOG be considered only a rough estimate. Keywords: Ark, kinds, created kinds, baraminology, birds Introduction As in mammals and amphibians, the state of avian $VSDUWRIWKH$UN(QFRXQWHUSURMHFW$QVZHUVLQ WD[RQRP\LVLQÁX['HVSLWHWKHLGHDORIQHDWO\QHVWHG Genesis initiated and funded research in an attempt hierarchies in taxonomy, it seems groups of birds to more clearly identify and enumerate the vertebrate are repeatedly “changing nests.” This is partially NLQGVWKDWZHUHSUHVHQWRQWKH$UN,QDQLQLWLDOSDSHU because where an animal is placed depends on which WKH FRQFHSW RI ELEOLFDO NLQGV ZDV GLVFXVVHG DQG D characteristics one chooses to consider. While many strategy to identify them was outlined (Lightner et al. had thought that molecular data would resolve these 6RPHRIWKHNH\SRLQWVDUHQRWHGEHORZ issues, in some cases it has exacerbated them. For this There is tremendous variety seen today in animal HVWLPDWHRIWKHDYLDQ$UNNLQGVWKHWD[RQRPLFVFKHPH OLIHDVFUHDWXUHVKDYHPXOWLSOLHGDQGÀOOHGWKHHDUWK presented online by the International Ornithologists’ since the Flood (Genesis 8:17). In order to identify 8QLRQ ,28 ZDVXVHG *LOODQG'RQVNHUD which modern species are related, being descendants 2012b and 2013). This list includes information on RI D VLQJOH NLQG LQWHUVSHFLÀF K\EULG GDWD LV XWLOL]HG extant and some recently extinct species. -

On the Origin and Evolution of Nest Building by Passerine Birds’
T H E C 0 N D 0 R r : : ,‘ “; i‘ . .. \ :i A JOURNAL OF AVIAN BIOLOGY ,I : Volume 99 Number 2 ’ I _ pg$$ij ,- The Condor 99~253-270 D The Cooper Ornithological Society 1997 ON THE ORIGIN AND EVOLUTION OF NEST BUILDING BY PASSERINE BIRDS’ NICHOLAS E. COLLIAS Departmentof Biology, Universityof California, Los Angeles, CA 90024-1606 Abstract. The object of this review is to relate nest-buildingbehavior to the origin and early evolution of passerinebirds (Order Passeriformes).I present evidence for the hypoth- esis that the combinationof small body size and the ability to place a constructednest where the bird chooses,helped make possiblea vast amountof adaptiveradiation. A great diversity of potential habitats especially accessibleto small birds was created in the late Tertiary by global climatic changes and by the continuing great evolutionary expansion of flowering plants and insects.Cavity or hole nests(in ground or tree), open-cupnests (outside of holes), and domed nests (with a constructedroof) were all present very early in evolution of the Passeriformes,as indicated by the presenceof all three of these basic nest types among the most primitive families of living passerinebirds. Secondary specializationsof these basic nest types are illustratedin the largest and most successfulfamilies of suboscinebirds. Nest site and nest form and structureoften help characterizethe genus, as is exemplified in the suboscinesby the ovenbirds(Furnariidae), a large family that builds among the most diverse nests of any family of birds. The domed nest is much more common among passerinesthan in non-passerines,and it is especially frequent among the very smallestpasserine birds the world over. -

Ecuador Trip Report Andes to the Amazon 5Th to 19Th September 2011 (15 Days)
Ecuador Trip Report Andes to the Amazon 5th to 19th September 2011 (15 days) Giant Antpitta by Luis Segura Tour Leader: Forrest Rowland Some of our tour highlights included: 1. ZigZag Heron 2. Giant Antpitta 3. Dark-backed Wood Quail 4. Lunulated Antbird 5. Toucan Barbet 6. Plate-billed Mountain Toucan 7. Rusty-belted Tapaculo 8. Orange-breasted Fruiteater 9. Long-tailed Potoo 10. Collared Puffbird RBT Ecuador Trip Report 2011 2 Tour Intro Ecuador is quite simply incredible! In the past 15 years, this humble country has become the most traversed and well-known of all South American nations by birders, and for good reason. Despite being no larger in size than the state of Colorado (USA), and occupying less than one-quarter the size of the Republic of Colombia, Ecuador has 30% more bird species than the whole of North America and only 16% less than Colombia. This is no doubt due to the fact that Ecuador’s political boundaries include some of the highest peaks in the Andean chain, perpetually snow-bound, and, of course, the humid rainforests of the Amazon, while two distinct Andean chains result in a dry Inter- Andean Valley with separate rainshadows on the outer slopes, and every habitat that such a varied and rich equatorial topography could possible harbor. The goal of this tour was to provide an introduction to these incredible birding zones. In most countries, 14 days would fall far short in terms of adequately representing such diversity: distances would be too great, travel times prohibitive and, quite often, infrastructure in rural South America just doesn’t allow for any visitors. -

Mammalian and Avian Diversity of the Rewa Head, Rupununi, Southern Guyana
Biota Neotrop., vol. 11, no. 3 Mammalian and avian diversity of the Rewa Head, Rupununi, Southern Guyana Robert Stuart Alexander Pickles1,2, Niall Patrick McCann1 & Ashley Peregrine Holland1 1Institute of Zoology, Zoological Society of London, Regent’s Park, London, NW1 4RY, School of Biosciences,Cardiff University, Museum Avenue, Cardiff, Wales, CF103AX Rupununi River Drifters, Karanambu Ranch, Lethem Post Office, Region 9, Rupununi Guyana 2Corresponding author: Robert Stuart Alexander Pickles, e-mail: [email protected] PICKLES, R.S.A., McCANN, N.P. & HOLLAND, A.L. Mammalian and avian diversity of the Rewa Head, Rupununi, Southern Guyana. Biota Neotrop. 11(3): http://www.biotaneotropica.org.br/v11n3/en/abstract?in ventory+bn00911032011 Abstract: We report the results of a short expedition to the remote headwaters of the River Rewa, a tributary of the River Essequibo in the Rupununi, Southern Guyana. We used a combination of camera trapping, mist netting and spot count surveys to document the mammalian and avian diversity found in the region. We recorded a total of 33 mammal species including all 8 of Guyana’s monkey species as well as threatened species such as lowland tapir (Tapirus terrestris), giant otter (Pteronura brasiliensis) and bush dog (Speothos venaticus). We recorded a minimum population size of 35 giant otters in five packs along the 95 km of river surveyed. In total we observed 193 bird species from 47 families. With the inclusion of Smithsonian Institution data from 2006, the bird species list for the Rewa Head rises to 250 from 54 families. These include 10 Guiana Shield endemics and two species recorded as rare throughout their ranges: the harpy eagle (Harpia harpyja) and crested eagle (Morphnus guianensis). -

TOP BIRDING LODGES of PANAMA with the Illinois Ornithological Society
TOP BIRDING LODGES OF PANAMA WITH IOS: JUNE 26 – JULY 5, 2018 TOP BIRDING LODGES OF PANAMA with the Illinois Ornithological Society June 26-July 5, 2018 Guides: Adam Sell and Josh Engel with local guides Check out the trip photo gallery at www.redhillbirding.com/panama2018gallery2 Panama may not be as well-known as Costa Rica as a birding and wildlife destination, but it is every bit as good. With an incredible diversity of birds in a small area, wonderful lodges, and great infrastructure, we tallied more than 300 species while staying at two of the best birding lodges anywhere in Central America. While staying at Canopy Tower, we birded Pipeline Road and other lowland sites in Soberanía National Park and spent a day in the higher elevations of Cerro Azul. We then shifted to Canopy Lodge in the beautiful, cool El Valle de Anton, birding the extensive forests around El Valle and taking a day trip to coastal wetlands and the nearby drier, more open forests in that area. This was the rainy season in Panama, but rain hardly interfered with our birding at all and we generally had nice weather throughout the trip. The birding, of course, was excellent! The lodges themselves offered great birding, with a fruiting Cecropia tree next to the Canopy Tower which treated us to eye-level views of tanagers, toucans, woodpeckers, flycatchers, parrots, and honeycreepers. Canopy Lodge’s feeders had a constant stream of birds, including Gray-cowled Wood-Rail and Dusky-faced Tanager. Other bird highlights included Ocellated and Dull-mantled Antbirds, Pheasant Cuckoo, Common Potoo sitting on an egg(!), King Vulture, Black Hawk-Eagle being harassed by Swallow-tailed Kites, five species of motmots, five species of trogons, five species of manakins, and 21 species of hummingbirds. -
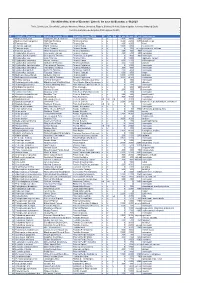
Ecuador Simple List Version 2020 Clements
Checklist of the birds of Ecuador / Lista de las aves del Ecuador, v. 08.2020 Freile, Brinkhuizen, Greenfield, Lysinger, Navarrete, Nilsson, Olmstead, Ridgely, Sánchez-Nivicela, Solano-Ugalde, Athanas, Ahlman & Boyla Comité Ecuatoriano de Registros Ornitológicos (CERO) ID Scientific_Clements_2019 English_Clements_2019 Español Ecuador CERO EC Con Gal Alt_min Alt_max Alt_ext Subespecies 1 Nothocercus julius Tawny-breasted Tinamou Tinamú Pechileonado x x 2300 3400 2100 monotypic 2 Nothocercus bonapartei Highland Tinamou Tinamú Serrano x x 1600 2200 3075 plumbeiceps 3 Tinamus tao Gray Tinamou Tinamú Gris x x 400 1600 kleei 4 Tinamus osgoodi Black Tinamou Tinamú Negro x x 1000 1400 hershkovitzi 5 Tinamus major Great Tinamou Tinamú Grande x x 0 700 1200, 1350 peruvianus, latifrons 6 Tinamus guttatus White-throated Tinamou Tinamú Goliblanco x x 200 400 900 monotypic 7 Crypturellus cinereus Cinereous Tinamou Tinamú Cinéreo x x 200 600 900 monotypic 8 Crypturellus berlepschi Berlepsch's Tinamou Tinamú de Berlepsch x x 0 400 900 monotypic 9 Crypturellus soui Little Tinamou Tinamú Chico x x 0 1200 nigriceps, harterti 10 Crypturellus obsoletus Brown Tinamou Tinamú Pardo x x 500 1100 chirimotanus? 11 Crypturellus undulatus Undulated Tinamou Tinamú Ondulado x x 200 600 yapura 12 Crypturellus transfasciatus Pale-browed Tinamou Tinamú Cejiblanco x x 0 1600 monotypic 13 Crypturellus variegatus Variegated Tinamou Tinamú Abigarrado x x 200 400 monotypic 14 Crypturellus bartletti Bartlett's Tinamou Tinamú de Bartlett x x 200 400 monotypic 15 Crypturellus