( Sebastes Schlegelii ) Under Changing Climate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Parallel Adaptations of Japanese Whiting, Sillago Japonica Under Temperature Stress
Parallel adaptations of Japanese whiting, Sillago japonica under temperature stress Zhiqiang Han1, Xinyu Guo2, Qun Liu2, Shanshan Liu2, Zhixin Zhang3, shijun xiao4, and Tianxiang Gao5 1Affiliation not available 2BGI-Shenzhen 3Tokyo University of Marine Science and Technology Graduate School of Marine Science and Technology 4Tibet Academy of Agricultural and Animal Husbandry Sciences 5Zhejiang Ocean University February 9, 2021 Abstract Knowledge about the genetic adaptations of various organisms to heterogeneous environments in the Northwestern Pacific remains poorly understood. The mechanism by which organisms adapt to temperature in response to climate change must be determined. We sequenced the whole genomes of Sillago japonica individuals collected from different latitudinal locations along the coastal waters of China and Japan to detect the possible thermal adaptations. A total of 5.48 million single nucleotide polymorphisms (SNPs) from five populations revealed a complete genetic break between the China and Japan groups. This genetic structure was partly attributed to geographic distance and local adaptation. Although parallel evolution within species is comparatively rare at the DNA level, the shared natural selection genes between two isolated populations (Zhoushan and Ise Bay/Tokyo Bay) indicated possible parallel evolution at the genetic level induced by temperature. Our result proved that the process of temperature selection on isolated populations is repeatable. Additionally, the candidate genes were functionally related to membrane fluidity in cold environments and the cytoskeleton in high-temperature environments. These results advance our understanding of the genetic mechanisms underlying the rapid adaptations of fish species. Projections of species distribution models suggested that China and Japan groups may have different responses to future climate changes: the former could expand, whereas the latter may contract. -

Book of Abstracts
PICES-2016 25 Year of PICES: Celebrating the Past, Imagining the Future North Pacific Marine Science Organization November 2-13, 2016 San Diego, CA, USA Table of Contents Notes for Guidance � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 5 Venue Floor Plan � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 6 List of Sessions/Workshops � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 9 Meeting Timetable � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 10 PICES Structure � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 12 PICES Acronyms � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 13 Session/Workshop Schedules at a Glance � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 15 List of Posters � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 47 Sessions and Workshops Descriptions � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 63 Abstracts Oral Presentations (ordered by days) � � � � � � � � � � � � � � � � � � � � � -

Southward Range Extension of the Goldeye Rockfish, Sebastes
Acta Ichthyologica et Piscatoria 51(2), 2021, 153–158 | DOI 10.3897/aiep.51.68832 Southward range extension of the goldeye rockfish, Sebastes thompsoni (Actinopterygii: Scorpaeniformes: Scorpaenidae), to northern Taiwan Tak-Kei CHOU1, Chi-Ngai TANG2 1 Department of Oceanography, National Sun Yat-sen University, Kaohsiung, Taiwan 2 Department of Aquaculture, National Taiwan Ocean University, Keelung, Taiwan http://zoobank.org/5F8F5772-5989-4FBA-A9D9-B8BD3D9970A6 Corresponding author: Tak-Kei Chou ([email protected]) Academic editor: Ronald Fricke ♦ Received 18 May 2021 ♦ Accepted 7 June 2021 ♦ Published 12 July 2021 Citation: Chou T-K, Tang C-N (2021) Southward range extension of the goldeye rockfish, Sebastes thompsoni (Actinopterygii: Scorpaeniformes: Scorpaenidae), to northern Taiwan. Acta Ichthyologica et Piscatoria 51(2): 153–158. https://doi.org/10.3897/ aiep.51.68832 Abstract The goldeye rockfish,Sebastes thompsoni (Jordan et Hubbs, 1925), is known as a typical cold-water species, occurring from southern Hokkaido to Kagoshima. In the presently reported study, a specimen was collected from the local fishery catch off Keelung, northern Taiwan, which represents the first specimen-based record of the genus in Taiwan. Moreover, the new record ofSebastes thompsoni in Taiwan represented the southernmost distribution of the cold-water genus Sebastes in the Northern Hemisphere. Keywords cold-water fish, DNA barcoding, neighbor-joining, new recorded genus, phylogeny, Sebastes joyneri Introduction On an occasional survey in a local fish market (25°7.77′N, 121°44.47′E), a mature female individual of The rockfish genusSebastes Cuvier, 1829 is the most spe- Sebastes thompsoni (Jordan et Hubbs, 1925) was obtained ciose group of the Scorpaenidae, which comprises about in the local catches, which were caught off Keelung, north- 110 species worldwide (Li et al. -

Looking Beyond the Mortality of Bycatch: Sublethal Effects of Incidental Capture on Marine Animals
Biological Conservation 171 (2014) 61–72 Contents lists available at ScienceDirect Biological Conservation journal homepage: www.elsevier.com/locate/biocon Review Looking beyond the mortality of bycatch: sublethal effects of incidental capture on marine animals a, a a,b b a Samantha M. Wilson ⇑, Graham D. Raby , Nicholas J. Burnett , Scott G. Hinch , Steven J. Cooke a Fish Ecology and Conservation Physiology Laboratory, Department of Biology and Institute of Environmental Sciences, Carleton University, Ottawa, ON, Canada b Pacific Salmon Ecology and Conservation Laboratory, Department of Forest and Conservation Sciences, University of British Columbia, Vancouver, BC, Canada article info abstract Article history: There is a widely recognized need to understand and reduce the incidental effects of marine fishing on Received 14 August 2013 non-target animals. Previous research on marine bycatch has largely focused on simply quantifying mor- Received in revised form 10 January 2014 tality. However, much less is known about the organism-level sublethal effects, including the potential Accepted 13 January 2014 for behavioural alterations, physiological and energetic costs, and associated reductions in feeding, growth, or reproduction (i.e., fitness) which can occur undetected following escape or release from fishing gear. We reviewed the literature and found 133 marine bycatch papers that included sublethal endpoints Keywords: such as physiological disturbance, behavioural impairment, injury, reflex impairment, and effects on RAMP reproduction, -

ORGANIC CHEMICAL TOXICOLOGY of FISHES This Is Volume 33 in The
ORGANIC CHEMICAL TOXICOLOGY OF FISHES This is Volume 33 in the FISH PHYSIOLOGY series Edited by Anthony P. Farrell and Colin J. Brauner Honorary Editors: William S. Hoar and David J. Randall A complete list of books in this series appears at the end of the volume ORGANIC CHEMICAL TOXICOLOGY OF FISHES Edited by KEITH B. TIERNEY Department of Biological Sciences University of Alberta Edmonton, Alberta Canada ANTHONY P. FARRELL Department of Zoology, and Faculty of Land and Food Systems The University of British Columbia Vancouver, British Columbia Canada COLIN J. BRAUNER Department of Zoology The University of British Columbia Vancouver, British Columbia Canada AMSTERDAM BOSTON HEIDELBERG LONDON NEW YORK OXFORD PARIS SAN DIEGO SAN FRANCISCO SINGAPORE SYDNEY TOKYO Academic Press is an imprint of Elsevier Academic Press is an imprint of Elsevier 32 Jamestown Road, London NW1 7BY, UK 225 Wyman Street, Waltham, MA 02451, USA 525 B Street, Suite 1800, San Diego, CA 92101-4495, USA Copyright r 2014 Elsevier Inc. All rights reserved The cover illustrates the diversity of effects an example synthetic organic water pollutant can have on fish. The chemical shown is 2,4-D, an herbicide that can be found in streams near urbanization and agriculture. The fish shown is one that can live in such streams: rainbow trout (Oncorhynchus mykiss). The effect shown on the left is the ability of 2,4-D (yellow line) to stimulate olfactory sensory neurons vs. control (black line) (measured as an electro- olfactogram; EOG). The effect shown on the right is the ability of 2,4-D to induce the expression of an egg yolk precursor protein (vitellogenin) in male fish. -
2018 Final LOFF W/ Ref and Detailed Info
Final List of Foreign Fisheries Rationale for Classification ** (Presence of mortality or injury (P/A), Co- Occurrence (C/O), Company (if Source of Marine Mammal Analogous Gear Fishery/Gear Number of aquaculture or Product (for Interactions (by group Marine Mammal (A/G), No RFMO or Legal Target Species or Product Type Vessels processor) processing) Area of Operation or species) Bycatch Estimates Information (N/I)) Protection Measures References Detailed Information Antigua and Barbuda Exempt Fisheries http://www.fao.org/fi/oldsite/FCP/en/ATG/body.htm http://www.fao.org/docrep/006/y5402e/y5402e06.htm,ht tp://www.tradeboss.com/default.cgi/action/viewcompan lobster, rock, spiny, demersal fish ies/searchterm/spiny+lobster/searchtermcondition/1/ , (snappers, groupers, grunts, ftp://ftp.fao.org/fi/DOCUMENT/IPOAS/national/Antigua U.S. LoF Caribbean spiny lobster trap/ pot >197 None documented, surgeonfish), flounder pots, traps 74 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G AndBarbuda/NPOA_IUU.pdf Caribbean mixed species trap/pot are category III http://www.nmfs.noaa.gov/pr/interactions/fisheries/tabl lobster, rock, spiny free diving, loops 19 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G e2/Atlantic_GOM_Caribbean_shellfish.html Queen conch (Strombus gigas), Dive (SCUBA & free molluscs diving) 25 not applicable not applicable Antigua & Barbuda EEZ none documented none documented A/G U.S. trade data Southeastern U.S. Atlantic, Gulf of Mexico, and Caribbean snapper- handline, hook and grouper and other reef fish bottom longline/hook-and-line/ >5,000 snapper line 71 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented N/I, A/G U.S. -

Toxic Effects of Ammonia Exposure on Growth Performance, Hematological
Shin et al. Fisheries and Aquatic Sciences (2016) 19:44 DOI 10.1186/s41240-016-0044-6 RESEARCH ARTICLE Open Access Toxic effects of ammonia exposure on growth performance, hematological parameters, and plasma components in rockfish, Sebastes schlegelii, during thermal stress Ki Won Shin2, Shin-Hu Kim1, Jun-Hwan Kim1, Seong Don Hwang2 and Ju-Chan Kang1* Abstract Rockfish, Sebastes schlegelii (mean length 14.53 ± 1.14 cm and mean weight 38.36 ± 3.45 g), were exposed for 4 weeks with the different levels of ammonia in the concentrations of 0, 0.1, 0.5, and 1.0 mg/L at 19 and 24 °C. The indicators of growth performance such as daily length gain, daily weight gain, condition factor, and hematosomatic index were significantly reduced by the ammonia exposure and high temperature. The ammonia exposure induced a significant decrease in hematological parameters, such as red blood cell (RBC) count, white blood cell (WBC) count, hemoglobin (Hb), and hematocrit (Ht), whose trend was more remarkable at 24 °C. Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were also notably decreased by the ammonia exposure. Blood ammonia concentration was considerably increased by the ammonia concentration exposure. In the serum components, the glucose, glutamic oxalate transaminase (GOT), and glutamic pyruvate transaminase (GPT) were substantially increased by the ammonia exposure, whereas total protein was significantly decreased. But, the calcium and magnesium were not considerably changed. Key words: Ammonia, Hematological parameters, Growth performance, Plasma components, Rockfish Background performance decrease, tissue erosion and degeneration, Ammonia is one of the nitrogenous wastes especially in immune suppression, and high mortality in aquatic ani- water. -
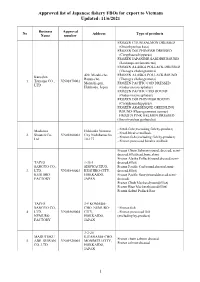
Approved List of Japanese Fishery Fbos for Export to Vietnam Updated: 11/6/2021
Approved list of Japanese fishery FBOs for export to Vietnam Updated: 11/6/2021 Business Approval No Address Type of products Name number FROZEN CHUM SALMON DRESSED (Oncorhynchus keta) FROZEN DOLPHINFISH DRESSED (Coryphaena hippurus) FROZEN JAPANESE SARDINE ROUND (Sardinops melanostictus) FROZEN ALASKA POLLACK DRESSED (Theragra chalcogramma) 420, Misaki-cho, FROZEN ALASKA POLLACK ROUND Kaneshin Rausu-cho, (Theragra chalcogramma) 1. Tsuyama CO., VN01870001 Menashi-gun, FROZEN PACIFIC COD DRESSED LTD Hokkaido, Japan (Gadus macrocephalus) FROZEN PACIFIC COD ROUND (Gadus macrocephalus) FROZEN DOLPHIN FISH ROUND (Coryphaena hippurus) FROZEN ARABESQUE GREENLING ROUND (Pleurogrammus azonus) FROZEN PINK SALMON DRESSED (Oncorhynchus gorbuscha) - Fresh fish (excluding fish by-product) Maekawa Hokkaido Nemuro - Fresh bivalve mollusk. 2. Shouten Co., VN01860002 City Nishihamacho - Frozen fish (excluding fish by-product) Ltd 10-177 - Frozen processed bivalve mollusk Frozen Chum Salmon (round, dressed, semi- dressed,fillet,head,bone,skin) Frozen Alaska Pollack(round,dressed,semi- TAIYO 1-35-1 dressed,fillet) SANGYO CO., SHOWACHUO, Frozen Pacific Cod(round,dressed,semi- 3. LTD. VN01840003 KUSHIRO-CITY, dressed,fillet) KUSHIRO HOKKAIDO, Frozen Pacific Saury(round,dressed,semi- FACTORY JAPAN dressed) Frozen Chub Mackerel(round,fillet) Frozen Blue Mackerel(round,fillet) Frozen Salted Pollack Roe TAIYO 3-9 KOMABA- SANGYO CO., CHO, NEMURO- - Frozen fish 4. LTD. VN01860004 CITY, - Frozen processed fish NEMURO HOKKAIDO, (excluding by-product) FACTORY JAPAN -

Multiple Fetal Nutritional Patterns Before Parturition in Viviparous Fish Sebastes Schlegelii (Hilgendorf, 1880)
ORIGINAL RESEARCH published: 14 January 2021 doi: 10.3389/fmars.2020.571946 Multiple Fetal Nutritional Patterns Before Parturition in Viviparous Fish Sebastes schlegelii (Hilgendorf, 1880) Du Tengfei 1,2,3†, Xiao Yongshuang 1,2,4†, Zhao Haixia 1,2,3, Zhou Li 1,2,3, Liu Qinghua 1,2, Wang Xueying 1,2, Li Jun 1,2,4*, Xu Shihong 1,2, Wang Yanfeng 1,2, Yu Jiachen 1,2,3, Wu Lele 1,2,3, Wang Yunong 1,2,3 and Gao Guang 1,2,3 1 The Key Laboratory of Experimental Marine Biology, Centre for Ocean Mega-Science, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China, 2 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao, China, 3 University of Chinese Academy of Sciences, Beijing, China, 4 Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), Guangzhou, China Sebastes schlegelii is a commercially important fish with a special viviparous reproductive system that is cultured in near-shore seawater net cages in East Asia. In the gonadal Edited by: development of the species, the gonad of males mature before those of females, which Antonio Trincone, mature after mating. Mating in male/female fishes occurs in October of each year. Then, Consiglio Nazionale delle Ricerche (CNR), Italy females undergoing oocyte maturation complete fertilization using stored sperm in March Reviewed by: of the following year. The pregnancy is completed when larvae are produced in the Angela Cuttitta, ovary. It has been reported that embryonic nutrient supply originates entirely from the National Research Council (CNR), Italy female viviparous reproductive systems. -

Chinese Red Swimming Crab (Portunus Haanii) Fishery Improvement Project (FIP) in Dongshan, China (August-December 2018)
Chinese Red Swimming Crab (Portunus haanii) Fishery Improvement Project (FIP) in Dongshan, China (August-December 2018) Prepared by Min Liu & Bai-an Lin Fish Biology Laboratory College of Ocean and Earth Sciences, Xiamen University March 2019 Contents 1. Introduction........................................................................................................ 5 2. Materials and Methods ...................................................................................... 6 2.1. Study site and survey frequency .................................................................... 6 2.2. Sample collection .......................................................................................... 7 2.3. Species identification................................................................................... 10 2.4. Sample measurement ................................................................................... 11 2.5. Interviews.................................................................................................... 13 2.6. Estimation of annual capture volume of Portunus haanii ............................. 15 3. Results .............................................................................................................. 15 3.1. Species diversity.......................................................................................... 15 3.1.1. Species composition .............................................................................. 15 3.1.2. ETP species ......................................................................................... -

Acta Botanica Brasilica - 35(1): 22-36
Acta Botanica Brasilica - 35(1): 22-36. January-March 2021. doi: 10.1590/0102-33062020abb0188 Predicting the potential distribution of aquatic herbaceous plants in oligotrophic Central Amazonian wetland ecosystems Aline Lopes1, 2* , Layon Oreste Demarchi2, 3 , Augusto Cesar Franco1 , Aurélia Bentes Ferreira2, 3 , Cristiane Silva Ferreira1 , Florian Wittmann2, 4 , Ivone Neri Santiago2 , Jefferson da Cruz2, 5 , Jeisiane Santos da Silva2, 3 , Jochen Schöngart2, 3 , Sthefanie do Nascimento Gomes de Souza2 and Maria Teresa Fernandez Piedade2, 3 Received: May 25, 2020 Accepted: November 13, 2020 ABSTRACT . Aquatic herbaceous plants are especially suitable for mapping environmental variability in wetlands, as they respond quickly to environmental gradients and are good indicators of habitat preference. We describe the composition of herbaceous species in two oligotrophic wetland ecosystems, floodplains along black-water rivers (igapó) and wetlands upon hydromorphic sand soils (campinarana) in the Parque Nacional do Jaú and the Reserva de Desenvolvimento Sustentável Uatumã in Central Amazonia, both protected areas. We tested for the potential distribution range (PDR) of the most frequent species of these ecosystems, which are the ones that occurred in at least two of the sampled wetlands, using species distribution models (SDMs). In total, 98 aquatic herbaceous species were recorded, of which 63 occurred in igapós and 44 in campinaranas. Most igapó species had ample PDRs across the Neotropics, while most campinaranas species were restricted to the Amazon Basin. These results are congruent with studies that described similar distribution patterns for tree and bird species, which emphasizes a high degree of endemism in Amazonian campinarana. However, we also found differences in the potential distribution of species between the two protected areas, indicating high environmental variability of oligotrophic ecosystems that deserve further investigation to develop effective measures for their conservation and protection. -

Diet Analysis of Black Rockfish (Sebastes Melanops) from Stomach Contents Off the Coast of Newport, Oregon
Diet analysis of Black Rockfish (Sebastes melanops) from stomach contents off the coast of Newport, Oregon by Renee Doran A THESIS submitted to Oregon State University Honors College in partial fulfillment of the requirements for the degree of Honors Baccalaureate of Science in Biology: Marine Biology Option (Honors Scholar) Presented November 16, 2020 Commencement June 2021 1 2 AN ABSTRACT OF THE THESIS OF Renee Doran for the degree of Honors Baccalaureate of Science in Biology: Marine Biology Option presented on November 16, 2020. Title: Diet analysis of Black Rockfish (Sebastes melanops) from stomach contents off the coast of Newport, Oregon Abstract approved:_____________________________________________________ Scott Heppell Black Rockfish are important commercial and recreational species and understanding their biology is useful in developing best management practices. A common means to assess diet in both freshwater and marine fishes is stomach content analysis, which can determine diet composition and infer foraging behaviors. We conducted a stomach content analysis on 263 Black Rockfish stomachs from fish caught off Newport, Oregon in March and June through September. We enumerated and identified prey taxa to determine an index of relative importance (IRI) for prey groups. We processed stomachs and recorded prey weight, number of prey, and prey variation per stomach and measured total (filleted) length and sex for each sample. We found crab megalopa had the highest IRI but were not significantly more important than other prey groups. We did not find a significant difference in the diet habits of male and female fish; although, female fish had on average higher prey weight, number of prey, and variety of prey per stomach.