Bond Activation by Unconventional Lewis Pairs by Jacob Geri A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NBO Applications, 2020
NBO Bibliography 2020 2531 publications – Revised and compiled by Ariel Andrea on Aug. 9, 2021 Aarabi, M.; Gholami, S.; Grabowski, S. J. S-H ... O and O-H ... O Hydrogen Bonds-Comparison of Dimers of Thiocarboxylic and Carboxylic Acids Chemphyschem, (21): 1653-1664 2020. 10.1002/cphc.202000131 Aarthi, K. V.; Rajagopal, H.; Muthu, S.; Jayanthi, V.; Girija, R. Quantum chemical calculations, spectroscopic investigation and molecular docking analysis of 4-chloro- N-methylpyridine-2-carboxamide Journal of Molecular Structure, (1210) 2020. 10.1016/j.molstruc.2020.128053 Abad, N.; Lgaz, H.; Atioglu, Z.; Akkurt, M.; Mague, J. T.; Ali, I. H.; Chung, I. M.; Salghi, R.; Essassi, E.; Ramli, Y. Synthesis, crystal structure, hirshfeld surface analysis, DFT computations and molecular dynamics study of 2-(benzyloxy)-3-phenylquinoxaline Journal of Molecular Structure, (1221) 2020. 10.1016/j.molstruc.2020.128727 Abbenseth, J.; Wtjen, F.; Finger, M.; Schneider, S. The Metaphosphite (PO2-) Anion as a Ligand Angewandte Chemie-International Edition, (59): 23574-23578 2020. 10.1002/anie.202011750 Abbenseth, J.; Goicoechea, J. M. Recent developments in the chemistry of non-trigonal pnictogen pincer compounds: from bonding to catalysis Chemical Science, (11): 9728-9740 2020. 10.1039/d0sc03819a Abbenseth, J.; Schneider, S. A Terminal Chlorophosphinidene Complex Zeitschrift Fur Anorganische Und Allgemeine Chemie, (646): 565-569 2020. 10.1002/zaac.202000010 Abbiche, K.; Acharjee, N.; Salah, M.; Hilali, M.; Laknifli, A.; Komiha, N.; Marakchi, K. Unveiling the mechanism and selectivity of 3+2 cycloaddition reactions of benzonitrile oxide to ethyl trans-cinnamate, ethyl crotonate and trans-2-penten-1-ol through DFT analysis Journal of Molecular Modeling, (26) 2020. -

ORGANIC CHEMISTRY Alkynes
University of Michigan new functional groups. (3) Developing novel routes for functionalizing readily available organic building blocks DEPARTMENT OF CHEMISTRY such as alkenes and alkynes. (4) Exploring the reduc- tive coupling of aldehydes and alkynes or enones and ORGANIC CHEMISTRY alkynes. Recent studies have demonstrated strategies involving redox isomerization to avoid the use of reduc- Graduate Program ing agents in processes of this type. (5) The discovery of new glycosylation methods and their application in collaborative projects involving enzymatic C-H oxida- tion reactions. Traditional Synthetic Novel C–H Bond Approach Functionalization Approach Fluorinated amino acids can be used to make super-stable “Tef- O O N O NH N lon” proteins; here the interior of a small protein is packed with the Michigan offers a diverse selection of research oppor- 2 Br 1. Br cat. PdII fluorous amino acid hexafluoroleucine (Marsh). O N tunities in Organic Chemistry with particular strengths Y Y Y N 2. NH3/MeOH Oxidant–X in Organometallic Chemistry, Organic Synthesis, Bioor- X X H 3. CH3I ganic Chemistry, and Organic Materials. Our innovative treatment of autoimmune diseases and cancer. (3) Anti-anxiety research rotation program allows students to explore Anti-convulsant Developing and applying chemical tools to study a range of exciting possibilities before choosing an Palladium-catalyzed C-H functionalization of an arene (Sanford). the role of oxidants as signaling molecules and the advisor. Specific research projects in each area are biological basis of aging. (4) Elucidating catalytic highlighted below. mechanisms and essential active site features of me- talloenzymes and ribozymes, including protein farnes- Organic Synthesis yltransferase, UDP-3-O-acyl-GlcNAC deacetylase, his- R3 H tone deacetylase and ribonuclease P. -

Specific Determination of Airborne Sulfates and Sulfuric Acid
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1977 Specific etD ermination of Airborne Sulfates and Sulfuric Acid. Purnendu Kumar Dasgupta Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Dasgupta, Purnendu Kumar, "Specific eD termination of Airborne Sulfates and Sulfuric Acid." (1977). LSU Historical Dissertations and Theses. 3152. https://digitalcommons.lsu.edu/gradschool_disstheses/3152 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -

Acids Lewis Acids and Bases Lewis Acids Lewis Acids: H+ Cu2+ Al3+ Lewis Bases
E5 Lewis Acids and Bases (Session 1) Acids November 5 - 11 Session one Bronsted: Acids are proton donors. • Pre-lab (p.151) due • Problem • 1st hour discussion of E4 • Compounds containing cations other than • Lab (Parts 1and 2A) H+ are acids! Session two DEMO • Lab: Parts 2B, 3 and 4 Problem: Some acids do not contain protons Lewis Acids and Bases Example: Al3+ (aq) = ≈ pH 3! Defines acid/base without using the word proton: + H H H • Cl-H + • O Cl- • • •• O H H Acid Base Base Acid . A BASE DONATES unbonded ELECTRON PAIR/S. An ACID ACCEPTS ELECTRON PAIR/S . Deodorants and acid loving plant foods contain aluminum salts Lewis Acids Lewis Bases . Electron rich species; electron pair donors. Electron deficient species ; potential electron pair acceptors. Lewis acids: H+ Cu2+ Al3+ “I’m deficient!” Ammonia hydroxide ion water__ (ammine) (hydroxo) (aquo) Acid 1 Lewis Acid-Base Reactions Lewis Acid-Base Reactions Example Metal ion + BONDED H H H + • to water H + • O O • • •• molecules H H Metal ion Acid + Base Complex ion surrounded by water . The acid reacts with the base by bonding to one molecules or more available electron pairs on the base. The acid-base bond is coordinate covalent. The product is a complex or complex ion Lewis Acid-Base Reaction Products Lewis Acid-Base Reaction Products Net Reaction Examples Net Reaction Examples 2+ 2+ + + Pb + 4 H2O [Pb(H2O)4] H + H2O [H(H2O)] Lewis acid Lewis base Tetra aquo lead ion Lewis acid Lewis base Hydronium ion 2+ 2+ 2+ 2+ Ni + 6 H2O [Ni(H2O)6] Cu + 4 H2O [Cu(H2O)4] Lewis acid Lewis base Hexa aquo nickel ion Lewis acid Lewis base Tetra aquo copper(II)ion DEMO DEMO Metal Aquo Complex Ions Part 1. -
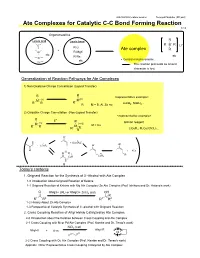
Ate Complexes for Catalytic C-C Bond Forming Reaction 1/13
2007/09/08 Literature seminer Tomoyuki Mashiko (M1 part) Ate Complexes for Catalytic C-C Bond Forming Reaction 1/13 Organometallics R Lewis acid Lewis base - B R B R Li+ R-Li + Ate complex R Al R-MgX etc R-Na etc Zn etc • Central metal is anionic. Cu The reaction proceeds as anionic character is lost. Generalization of Reaction Pathways for Ate Complexes 1) Non-Oxidative Charge Cancellation (Ligand Transfer) R R <representative example> M- (n) M(n) R R R LiAlH , NaBH .. R- M = B, Al, Zn etc 4 4. 2) Oxidative Charge Cancellation (Non-Ligand Transfer) <representative example> R + E R Gilman reagent M- (n) (n+2) R R M M = Cu R E LiCuR , R Cu(CN)Li .. R 2 2 2. O- Ⅱ + + [LiCuR2] - Ⅰ - O + LiCuR2 O Ⅰ O Li + RCu O R Ⅲ Ⅰ R CuR CuR2 0 Today's contents 1. Grignard Reaction for the Synthesis of 3°-Alcohol with Ate Complex 1-0 Introduction about Grignard Reaction of Ketone 1-1 Grignard Reaction of Ketone with Mg Ate Complex/ Zn Ate Complex (Prof. Ishihara and Dr. Hatano's work) O RMgX+ 2RLi or RMgCl+ ZnCl2 (cat) OH R R1 R2 R1 R2 1-2 History About Zn Ate Complex 1-3 Perspective of Catalytic Synthesis of 3°-alcohol with Grignard Reaction 2. Cross Coupling Reaction of Alkyl Halide Catalyzed by Ate Complex 2-0 Introduction about the Relation between Cross Coupling and Ate Complex 2-1 Cross Coupling with Ni or Pd Ate Complex (Prof. Kambe and Dr. Terao's work) NiCl2 (cat) Alkyl-R Alkyl-X + Ⅱ R-m Ni 2-2 Cross Coupling with Cu Ate Complex (Prof. -

Lesson 21: Acids & Bases
Lesson 21: Acids & Bases - Far From Basic Lesson Objectives: • Students will identify acids and bases by the Lewis, Bronsted-Lowry, and Arrhenius models. • Students will calculate the pH of given solutions. Getting Curious You’ve probably heard of pH before. Many personal hygiene products make claims about pH that are sometimes based on true science, but frequently are not. pH is the measurement of [H+] ion concentration in any solution. Generally, it can tell us about the acidity or alkaline (basicness) of a solution. Click on the CK-12 PLIX Interactive below for an introduction to acids, bases, and pH, and then answer the questions below. Directions: Log into CK-12 as follows: Username - your Whitmore School Username Password - whitmore2018 Questions: Copy and paste questions 1-3 in the Submit Box at the bottom of this page, and answer the questions before going any further in the lesson: 1. After dragging the small white circles (in this simulation, the indicator papers) onto each substance, what do you observe about the pH of each substance? 2. If you were to taste each substance (highly inadvisable in the chemistry laboratory!), how would you imagine they would taste? 3. Of the three substances, which would you characterize as acid? Which as basic? Which as neutral? Use the observed pH levels to support your hypotheses. Chemistry Time Properties of Acids and Bases Acids and bases are versatile and useful materials in many chemical reactions. Some properties that are common to aqueous solutions of acids and bases are listed in the table below. Acids Bases conduct electricity in solution conduct electricity in solution turn blue litmus paper red turn red litmus paper blue have a sour taste have a slippery feeling react with acids to create a neutral react with bases to create a neutral solution solution react with active metals to produce hydrogen gas Note: Litmus paper is a type of treated paper that changes color based on the acidity of the solution it comes in contact with. -

Document 4. Memòria D'activitats 2019
MEMÒRIA D’ACTIVITATS FUNDACIÓ INSTITUT CATALÀ D’INVESTIGACIÓ QUÍMICA ANY 2019 Memòria d’Activitats ICIQ 2019 2 Memòria d’Activitats ICIQ 2019 Sumari 1. L’Institut – visió general ......................................................................................................... 5 2. Governança i organització ..................................................................................................... 6 2.1. Governança .............................................................................................................. 6 2.2. Organització ............................................................................................................. 8 2.3. Àrea de Recerca ..................................................................................................... 11 3. Recursos humans ................................................................................................................. 16 3.1. Pla de formació ...................................................................................................... 18 4. Resultats de recerca ............................................................................................................ 21 4.1. Publicacions científiques ........................................................................................ 21 4.1.1. Anàlisi bibliomètric .................................................................................. 21 4.1.2. Publicacions en portades o contraportades de revistes ......................... 26 4.2. Recerca destacada ................................................................................................ -

Clark, Hobart, and Neu 1995
Waste Isolation Pilot Plant Compliance Certification Application Reference 135 Clark, D.L., D.E. Hobart, and M.P. Neu. 1995. Actinide Carbonate Complexes and Their Importance in Actinide Environmental Chemistry, Chem Revs. Vol. 95; 25-48. Submitted in accordance with 40 CFR $194.13, Submission of Reference Materials. Carera, ;., Neurcan. 3.p.. - 986 "Est~rncaonoi ?x:ier Parcrneters L'nae; Panslent anc' S:eadu Sco:z ~oi~icions,2. Unlacaness, Stc;z.:ird, and solucion ftiqo:ithrns." 9. Clark, D.L.. Floba~.D.E., Neu, M.P.. 1995 '9ctinicz Carbonate Complexes ana Thslr Irnporcccca in Ect;nide Environmencai G,~mrsny."=?em Revs. ',Jot. 05, 25-48. ON ' ; x 28.C3 398.00 Cleveland, J.M.. i 9* nGit:calRevleu of Plutonium Eov~lbrioof Environrnencal Concern. In Moueirng in Equmus S;lstms: Smrct:on, So~ubrlrtuanu tlnq of t9e Emencan Chernrcc~Societu, Pdiarnr Beacn, Fi, Series: 3521 -336. Cti~- ; x CLO.CC 220.30 . - , I. 2av1s.G.B.. Jcnnsco i 984 'Ccxxenc on Cmcamincnc Tianspor: :n fracturad PONS Media: fcr s Sjscern cS ~rciielFrcc:vres" 5y SLG~C~U,C.A., and Fmd, E.O.,' Rasc~rcesRzsecrcn, \jot. 23,i\.'o. 9. s?. : 321 - 1 322, Szpt. ., -- ? 984. 1 Qtl; I i x i 3.:: ' 48.50 , , -. - 4 Actinide Carbonate Complexes and Their Importance in Actinide Environmental Chemistry I David L. Clark,'~~~David E. Hobart,lb and Mary P. Neda Chemical Science and Technology Division, Los Alamas National Laboratory, Los Alamos, Mw Me& 87545, l?eThe Sciems DMm, Lawrence Berkeley Laboratory, BeBerky, California 94720, and UE G. T. Seabog imWe for Transactinium Scienae, I Livemre, California 94551 I Received May 16, 1994 (Revised Manuscript ReceM September 16, 1994) Table 1. -

Acids and Bases
self study Most of this is probably background from a prior chemistry course; some near the end is GG325 review Aqueous Inorganic Geochemistry of Natural Waters Three important topics: 1. Acids, bases and the aqueous CO 2 system 2. Behavior of ions in aqueous solution 3. Quantifying aqueous solubility and total dissolved solids (TDS) Pease read Manahan chapter 3 and review chapter 28 (6 th Ed) for next week GG425 Wk2, S2016 Acids and Bases GG425 Wk2, S2016 1 1. Two Types of Acids and Bases: a. Brönstead acids and bases contain H + and OH - HCl ↔ H + + + Cl- (acid) NaOH ↔ Na + + OH - (base). + - water is acid and base simultaneously, H 2O ↔ H + OH . pure water and acid neutral aqueous solutions have equal amounts of H + and OH -. b. Lewis acids and bases Brönstead acids can be thought of as electron deficient ions and bases as electron excessive ions , which provides a different perspective on acidity/basisity that we can extend to other (non protic and non hydroxyl) compounds. We call this the Lewis acid/base concept. GG425 Wk2, S2016 Brönstead acidity: Kw is the equilibrium constant for the dissociation of water. + - H2O ↔ H + OH + - -14 Kw = [H ][OH ] = 10 at 25 EC Kw has a slight temperature dependence: Temp ( EC) Kw 0 10 -14.94 less dissociated 25 10 -14 60 10 -13.02 more dissociated GG425 Wk2, S2016 2 Brönstead acidity: + - H2O ↔ H + OH + - Kw = [H ][OH ] An acid neutral solution always has [H +] = [OH -]. Setting [H +] = x, yields x 2= 10 -14 x = 10 -7 = moles of H + in this solution. -

The Reactivity of Hydrogen and Carbon Dioxide Mediated by Main Group Compounds
THE REACTIVITY OF HYDROGEN AND CARBON DIOXIDE MEDIATED BY MAIN GROUP COMPOUNDS A dissertation submitted to Imperial College London for the degree of Doctor of Philosophy By Thomas James Herrington MChem (Oxon), Imperial College London May 2014 CID: 00467189 Supervised By Dr Andrew Ashley & Dr George Britovsek Chemistry Department 1 ABSTRACT The Reactivity of Hydrogen and Carbon Dioxide Mediated by Main Group Compounds The focus of this thesis has been the design and synthesis of new frustrated Lewis pair (FLP) systems which from structural modifications retain their ability to activate H2/CO2, while displaying differing reactivity modes. Chapter Two describes the first practical synthesis of tris[3,5- bis(trifluoromethyl)phenyl]borane (BArF18). Gutmann-Beckett Lewis acidity measurements reveal that this borane is a more powerful Lewis acid than B(C6F5)3, but it nevertheless is found to bind H2O much more reversibly than B(C6F5)3. The BArF18/2,2,6,6- tetramethylpiperidine (TMP) FLP provides a rare example of H2 activation in Et2O solvent, in which the borohydride salt has been structurally characterised by X-ray crystallography. A – novel bridging borohydride [-H(BArF18)2] was revealed, which contrasts to the characteristic terminal borohydrides formed by other borane based mediated FLP systems. Chapter Three details the design of fluorinated trisalkylboranes including B[CH(C6F5)2]3 which has been synthesised for the first time. This borane has been structurally characterised using X-ray crystallography and displays hydrogen bonding interactions between the ortho fluorines on each aryl ring and the adjacent CH proton. Interestingly, and despite this borane showing no Lewis acidity using Gutmann-Beckett and Childs techniques, the B[CH(C6F5)2]3/TMP FLP provides a rare example of H2 activation in THF solvent. -

Group 16 Elements in Frustrated Lewis Pair Chemistry
Group 16 Elements in Frustrated Lewis Pair Chemistry by Fu An Tsao A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Department of Chemistry University of Toronto © Copyright by Fu An Tsao 2018 Group 16 Elements in Frustrated Lewis Pair Chemistry Fu An Tsao Doctor of Philosophy Department of Chemistry University of Toronto 2018 Abstract Frustrated Lewis Pairs (FLPs) describe combinations of sterically encumbered Lewis acids and bases that do not form classical adducts. This unquenched reactivity has been shown to activate a wide plethora of small molecules, including H2, CO2, alkynes and ketones. While many different main group compounds have been applied in this context, studies of group 16 elements in FLP chemistry remain scarce. The main objective of this dissertation is to expand the scope of FLP chemistry to include group 16 elements, as they can function both as Lewis bases and as Lewis acids. The first portion of this dissertation focuses on the syntheses and reactivity studies of tellurium-boron heterocycles, wherein tellurium acts as a Lewis base. 1,1-carboboration of tellurium-substituted acetylides was shown to proceed smoothly at room temperature, leading to the formation of Te/B intramolecular FLP and 1,4-telluraborine. Several 1,4- telluraborines were found to have mild to high aromaticity as manifested by their unusual stability against oxygen and moisture. They can also undergo a number of unique reactions, including FLP-type alkyne exchange reactions and selective protonolysis of the exocyclic B–C bond. These findings allowed for the facile derivatization of this new class of compounds. -

Development of Novel Methodologies in Organic Synthesis Based on Ate Complex Formation
No.171 No.171 ResearchResearch ArticleArticle Development of Novel Methodologies in Organic Synthesis Based on Ate Complex Formation Keiichi Hirano1* and Masanobu Uchiyama1,2* 1 Graduate School of Pharmaceutical Sciences, The University of Tokyo,7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan 2 Elements Chemistry Laboratory, RIKEN,2-1 Hirosawa, Wako-shi, Saitama 351-0198, Japan E-mail: [email protected]; [email protected] Abstract: We have been focusing on development of novel synthetic methodologies based on ate complex formation. By fine-tuning of coordination environments of various main-group metals (Zn, Al, and B) as well as transition metal (Cu), a wide range of carbon-carbon bond and carbon-heteroatom bond (C–I, C–O, C–N, C–H, C–Si, and C–B) formation reactions have been realized in highly regio- and chemoselective manner taking advantage of characteristics of the elements. Keywords: Ate complex, Halogen-metal exchange, Deprotonative metalation, Chemoselectivity, Regioselectivity 1. Introduction 2. Ate Complexes Ate complexes are known to be anionic organometallic Discovery of the first mono-anion type zincate, Na[ZnEt3], complexes, whose central metals have increased valence by by James A. Wanklyn dates back to 1859,1) and even di-anion accepting Lewis basic ligands to their vacant orbitals. They type zincate, Li2[ZnMe4], was already reported by Dallas T. are attractive chemical species offering tunable reactivity due Hurd in 1948.2) Zincates experienced a long blank after these to their flexible choices of central metals, counter cations, important findings before they started to attract attentions and coordination environments.