The Making of Stars 'R'
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Origins of Life in the Universe Zackary Johnson
11/4/2007 Origins of Life in the Universe Zackary Johnson OCN201 Fall 2007 [email protected] Zackary Johnson http://www.soest.hawaii.edu/oceanography/zij/education.html Uniiiversity of Hawaii Department of Oceanography Class Schedule Nov‐2Originsof Life and the Universe Nov‐5 Classification of Life Nov‐7 Primary Production Nov‐9Consumers Nov‐14 Evolution: Processes (Steward) Nov‐16 Evolution: Adaptation() (Steward) Nov‐19 Marine Microbiology Nov‐21 Benthic Communities Nov‐26 Whale Falls (Smith) Nov‐28 The Marine Food Web Nov‐30 Community Ecology Dec‐3 Fisheries Dec‐5Global Ecology Dec‐12 Final Major Concepts TIMETABLE Big Bang! • Life started early, but not at the beginning, of Earth’s Milky Way (and other galaxies formed) history • Abiogenesis is the leading hypothesis to explain the beginning of life on Earth • There are many competing theories as to how this happened • Some of the details have been worked out, but most Formation of Earth have not • Abiogenesis almost certainly occurred in the ocean 20‐15 15‐94.5Today Billions of Years Before Present 1 11/4/2007 Building Blocks TIMETABLE Big Bang! • Universe is mostly hydrogen (H) and helium (He); for Milky Way (and other galaxies formed) example –the sun is 70% H, 28% He and 2% all else! Abundance) e • Most elements of interest to biology (C, N, P, O, etc.) were (Relativ 10 produced via nuclear fusion Formation of Earth Log at very high temperature reactions in large stars after Big Bang 20‐13 13‐94.7Today Atomic Number Billions of Years Before Present ORIGIN OF LIFE ON EARTH Abiogenesis: 3 stages Divine Creation 1. -

Quantifying the Origins of Life on a Planetary Scale
Quantifying the origins of life on a planetary scale Caleb Scharfa,1 and Leroy Croninb,1 aColumbia Astrobiology Center, Columbia Astrophysics Laboratory, New York, NY 10027; and bSchool of Chemistry, University of Glasgow, Glasgow, G12 8QQ, United Kingdom Edited by Neta A. Bahcall, Princeton University, Princeton, NJ, and approved May 17, 2016 (received for review November 23, 2015) A simple, heuristic formula with parallels to the Drake Equation is currently impossible to construct a first principles quantitative introduced to help focus discussion on open questions for the origins estimate of an abiogenesis probability for the young Earth. It is of life in a planetary context. This approach indicates a number of also the case that we typically conflate the idea of “microabio- areas where quantitative progress can be made on parameter genesis”— namely, a highly localized assembly of molecular estimation for determining origins of life probabilities, based on structures that meets the minimum criterion for a living system— constraints from Bayesian approaches. We discuss a variety of “micro- with “macro-” or planet-wide abiogenesis. In other words the idea scale” factors and their role in determining “macroscale” abiogenesis of life arising on a planet is treated as a singular event rather than probabilities on suitable planets. We also propose that impact ejecta a possible accumulation of many microscale events, each of which exchange between planets with parallel chemistries and chemical evo- might be considered an origin event in its own right, and are ar- lution could in principle amplify the development of molecular com- guably more accessible through laboratory or modeling investi- plexity and abiogenesis probabilities. -
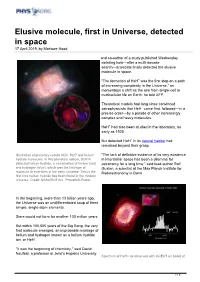
Elusive Molecule, First in Universe, Detected in Space 17 April 2019, by Marlowe Hood
Elusive molecule, first in Universe, detected in space 17 April 2019, by Marlowe Hood and co-author of a study published Wednesday detailing how—after a multi-decade search—scientists finally detected the elusive molecule in space. "The formation of HeH+ was the first step on a path of increasing complexity in the Universe," as momentous a shift as the one from single-cell to multicellular life on Earth, he told AFP. Theoretical models had long since convinced astrophysicists that HeH+ came first, followed—in a precise order—by a parade of other increasingly complex and heavy molecules. HeH+ had also been studied in the laboratory, as early as 1925. But detected HeH+ in its natural habitat had remained beyond their grasp. Illustration of planetary nebula NGC 7027 and helium "The lack of definitive evidence of its very existence hydride molecules. In this planetary nebula, SOFIA in interstellar space has been a dilemma for detected helium hydride, a combination of helium (red) astronomy for a long time," said lead author Rolf and hydrogen (blue), which was the first type of Gusten, a scientist at the Max Planck Institute for molecule to ever form in the early universe. This is the Radioastronomy in Bonn. first time helium hydride has been found in the modern universe. Credit: NASA/SOFIA/L. Proudfit/D.Rutter In the beginning, more than 13 billion years ago, the Universe was an undifferentiated soup of three simple, single-atom elements. Stars would not form for another 100 million years. But within 100,000 years of the Big Bang, the very first molecule emerged, an improbable marriage of helium and hydrogen known as a helium hydride ion, or HeH+. -

The Dissipative Photochemical Origin of Life: UVC Abiogenesis of Adenine
entropy Article The Dissipative Photochemical Origin of Life: UVC Abiogenesis of Adenine Karo Michaelian Department of Nuclear Physics and Applications of Radiation, Instituto de Física, Universidad Nacional Autónoma de México, Circuito Interior de la Investigación Científica, Cuidad Universitaria, Mexico City, C.P. 04510, Mexico; karo@fisica.unam.mx Abstract: The non-equilibrium thermodynamics and the photochemical reaction mechanisms are described which may have been involved in the dissipative structuring, proliferation and complex- ation of the fundamental molecules of life from simpler and more common precursors under the UVC photon flux prevalent at the Earth’s surface at the origin of life. Dissipative structuring of the fundamental molecules is evidenced by their strong and broad wavelength absorption bands in the UVC and rapid radiationless deexcitation. Proliferation arises from the auto- and cross-catalytic nature of the intermediate products. Inherent non-linearity gives rise to numerous stationary states permitting the system to evolve, on amplification of a fluctuation, towards concentration profiles providing generally greater photon dissipation through a thermodynamic selection of dissipative efficacy. An example is given of photochemical dissipative abiogenesis of adenine from the precursor HCN in water solvent within a fatty acid vesicle floating on a hot ocean surface and driven far from equilibrium by the incident UVC light. The kinetic equations for the photochemical reactions with diffusion are resolved under different environmental conditions and the results analyzed within the framework of non-linear Classical Irreversible Thermodynamic theory. Keywords: origin of life; dissipative structuring; prebiotic chemistry; abiogenesis; adenine; organic molecules; non-equilibrium thermodynamics; photochemical reactions Citation: Michaelian, K. The Dissipative Photochemical Origin of MSC: 92-10; 92C05; 92C15; 92C40; 92C45; 80Axx; 82Cxx Life: UVC Abiogenesis of Adenine. -

NASA Armstrong X-Press, May 2019
National Aeronautics and Space Administration Volume 61 Number 4 May 2019 Molecule is found Long search for the universe’s first type of molecule is over By Kassandra Bell and Alison Hawkes USRA and NASA Ames Public Affairs The first type of molecule that ever formed in the universe has been detected in space for the first time, after decades of searching. Scientists discovered its signature in the Milky Way Galaxy using the world’s largest airborne observatory, NASA’s Stratospheric Observatory for Infrared Astronomy, or SOFIA, as the aircraft flew high above the Earth’s surface and pointed its sensitive instruments out into the cosmos. NASA/ESA/Hubble Processing: Judy Schmidt When the universe was still very young, only a few kinds of atoms This image of planetary nebula NGC 7027, with an illustration of helium existed. Scientists believe that around 100,000 years after the big bang, hydride molecules, shows where SOFIA detected helium hydride, a combination helium and hydrogen combined to make a molecule called helium hydride of helium (red) and hydrogen (blue). It was the first type of molecule to ever for the first time. Helium hydride should be present in some parts of the form in the early universe. This is the first time helium hydride has been found modern universe, but it has never been detected in space until now. in the modern universe. SOFIA found modern helium hydride in a planetary nebula, a remnant of what was once a sun-like star. Located 3,000 light-years away near the constellation Cygnus, this planetary nebula, called NGC 7027, has conditions that allow this mystery molecule to form. -

Image of Planetary Nebula NGC 7027 with Illustration of Helium Hydride Molecules
National Aeronautics and Space Administration Image of planetary nebula NGC 7027 with illustration of helium hydride molecules. In this planetary nebula, SOFIA detected helium hydride, a combination of helium (red) and hydrogen (blue), which was the first type of molecule to ever form in the early universe. This is the first time helium hydride has been found in the modern universe. Credit: NASA/ESA/Hubble Processing: Judy Schmidt The Universe’s First Type of Molecule Is Found at Last After decades of searching, the first type of molecule that Helium hydride is a finicky molecule. Helium itself is a noble ever formed in the universe has been detected in space for gas making it very unlikely to combine with any other kind of the first time. Scientists discovered its signature in our own atom. But in 1925, scientists were able to create the molecule galaxy using the world’s largest airborne observatory, NASA’s in a laboratory by coaxing the helium to share one of its elec- Stratospheric Observatory for Infrared Astronomy, or SOFIA, trons with a hydrogen ion. as the aircraft flew high above the Earth’s surface and pointed its sensitive instruments out into the cosmos. Then, in the late 1970s, scientists studying the planetary nebula called NGC 7027 thought that this environment might be just When the universe was still very young, only a few kinds of right to form helium hydride. Ultraviolet radiation and heat from atoms existed. Scientists believe that around 100,000 years the aging star create conditions suitable for helium hydride to after the big bang, helium and hydrogen combined to make form. -

The Agb Newsletter
THE AGB NEWSLETTER An electronic publication dedicated to Asymptotic Giant Branch stars and related phenomena Official publication of the IAU Working Group on Abundances in Red Giants No. 226 — 4 May 2016 http://www.astro.keele.ac.uk/AGBnews Editors: Jacco van Loon, Ambra Nanni and Albert Zijlstra Editorial Dear Colleagues, It is our pleasure to present you the 226th issue of the AGB Newsletter. Let us know if you can’t find your favourite topic! Have a look at the announcement at the back of the newsletter, for a special issue of Geochimica et Cosmochimica Acta to commemorate Ernst Zinner. The next issue is planned to be distributed around the 1st of June. Editorially Yours, Jacco van Loon, Ambra Nanni and Albert Zijlstra Food for Thought This month’s thought-provoking statement is: Are intergalactic PNe isolated? Reactions to this statement or suggestions for next month’s statement can be e-mailed to [email protected] (please state whether you wish to remain anonymous) 1 Refereed Journal Papers Chemical abundances and kinematics of barium stars D.B. de Castro1, C.B. Pereira1, F. Roig1, E. Jilinski1,2, N.A. Drake1,3, C. Chavero1,4,5 and J.V. Sales Silva1 1Observatorio Nacional/MCTI, Brazil 2Pulkovo Observatory, Russian Academy of Sciences, Russia 3Saint Petersburg State University, Russia 4Observatorio Astronomico de C´ordoba, Argentina 5Consejo Nacional de Investigaciones Cient´ıficas y T´ecnicas, Argentina In this paper we present an homogeneous analysis of photospheric abundances based on high-resolution spectroscopy of a sample of 182 barium stars and candidates. -

Spatial Distribution and Kinematics of the Molecular Material Associated with Η Carinae
Spatial distribution and kinematics of the molecular material associated with η Carinae Laurent Loinard1;2, Tomasz Kami´nski3, Paolo Serra4, Karl M. Menten1, Luis A. Zapata2, and Luis F. Rodr´ıguez2 Received ; accepted 1Max Planck Institut f¨urRadioastronomie, Auf dem H¨ugel69, 53121 Bonn, Germany arXiv:1606.04575v2 [astro-ph.SR] 12 Sep 2016 ([email protected]) 2Instituto de Radioastronom´ıay Astrof´ısica,Universidad Nacional Aut´onomade M´exico Apartado Postal 3-72, 58090, Morelia, Michoac´an,Mexico 3ESO, Alonso de C´ordova 3107, Vitacura, Casilla, 19001, Santiago, Chile 4CSIRO Astronomy, Space Science, Australia Telescope National Facility, PO Box 76, Epping, NSW 1710, Australia { 2 { ABSTRACT Single-dish sub-millimeter observations have recently revealed the existence of a substantial, chemically peculiar, molecular gas component located in the innermost circumstellar environment of the very massive luminous blue variable star η Carinae. Here, we present 500-resolution interferometric observations of the 1!0 rotational transition of hydrogen cyanide (HCN) obtained with the Australia Telescope Compact Array (ATCA) toward this star. The emission is concentrated in the central few arcseconds around η Carinae and shows a clear 150 km s−1 velocity gradient running from west-north-west (blue) to east- south-east (red). Given the extent, location, and kinematics of this molecular material, we associate it with the complex of dusty arcs and knots seen in mid- infrared emission near the center of the Homunculus nebula. Indeed, the shielding provided by this dust could help explain how molecules survive in the presence of the intense UV radiation field produced by η Carinae. -

Stellar Atmospheres by R
Stellar Atmosphere Volume 푉= (Area) (length) Collision = cross section 휎 ⋅ ℓ = 휎 퓋푡 Relative speed 퓋 Number of particles 푁 Number density 푛 = 푁Τ푉 # of collisions = # of (other) particles in the volume = 푁 = 푛 휎 퓋 t # of collisions per unit time = 푁 Τ푡 = 푛 휎 퓋 Time between 2 consecutive collisions (푁=1) (mean-free time), 푡col = 1 Τ (푛 휎 퓋) Distance between 2 consecutive collisions (푁=1) (mean-free path), ℓcol = 퓋푡col = 1 Τ (푛 휎 ) In general “encounters” between particles, or between a particle and a photon. The “cross section” is the key. 2 Opacity −1 ′ 2 −1 −3 휅 cm = 휅 휌 cm g ⋅ g cm = 푛푖 휎푖 푖 Recall that 휅 푑푠 is the optical depth If 휅휈 is frequency independent 휅 gray atmosphere Usually an average opacity is used 휅휈 퐵휈 푑휈 Planck opacity average of frequency 휅P = 퐵 푑휈 dependent opacity weighted by Planck function 휈 Svein Rosseland −1 Rosseland opacity weighted 휅휈 푢휈 푑휈 by 푇 derivative, averaging 1Τ휅휈 1Τ휅R = 푢휈 푑휈 푢 휈, 푇 = 휕퐵휈 푇 Τ휕푇 3 푎+푏+푐 Arithmetic average, , e.g., 1 + 4 + 4 Τ3 = 3 3 Geometric average, 3 푎 ∙ 푏 ∙ 푐, e.g., 3 1 × 4 × 4 ≈ 2.52 1Τ푎 + 1Τ푏 + 1Τ푐 −1 Harmonic average, one of the Pythagorean means, , 3 1Τ1 + 1Τ4 + 1Τ4 −1 e.g., = 2 3 4 Bound-bound absorption Excitation of an electron of an atom to a higher energy state by the absorption of a photon. The excited atom then will be de-excited spontaneously, emitting a photon, or by collision with another particle. -

Excited States of Molecules in Strong Uniform and Non-Uniform Magnetic fields Sangita Sen,1, A) Kai K
Excited states of molecules in strong uniform and non-uniform magnetic fields Sangita Sen,1, a) Kai K. Lange,1 and Erik I. Tellgren1, b) Hylleraas Centre for Quantum Molecular Sciences, Department of Chemistry, University of Oslo, P.O. Box 1033 Blindern, N-0315 Oslo, Norway (Dated: 1 February 2019) This paper reports an implementation of Hartree-Fock linear response with complex orbitals for computing electronic spectra of molecules in a strong external magnetic fields. The implementation is completely general, allowing for spin-restricted, spin-unrestricted, and general two-component reference states. The method is applied to small molecules placed in strong uniform and non-uniform magnetic fields of astrochemical importance at the Random Phase Approximation level of theory. For uniform fields, where comparison is possible, the spectra are found to be qualitatively similar to those recently obtained with equation of motion coupled cluster theory. We also study the behaviour of spin-forbidden excitations with progressive loss of spin symmetry induced by non-uniform magnetic fields. Finally, the equivalence of length and velocity gauges for oscillator strengths when using complex orbitals is investigated and found to hold numerically. I. INTRODUCTION employed to enforce gauge-origin invariance and accel- erate basis set convergence27–30. With ordinary Gaus- External magnetic fields can dramatically affect the sians it becomes necessary to use very large basis sets to 31–35 electronic structure of atoms and molecules when approach gauge-origin invariance . An implementa- the field interaction strengths are comparable to the tion of integral evaluation for the LAOs which are plane- 36–39 Coulomb interaction1,2. -

Helium Hydride
NATURE’S MARVELS Universe’s First Molecule Found Helium Hydride Kirti Chhabra Image of planetary nebula NGC 7027 with an illustration of Helium Hydride molecules Image Credit: NASA/ESA/Hubble 60 | Science Reporter | August 2019 60 | Science Reporter | August 2019 SOFIA is a modified Boeing 747SP aircraft Image Credit: NASA/Jim Ross HE universe today is filled with stars, planets and share one of its electrons with a hydrogen ion. But the galaxies, but what came before? After the big bang, existence of Helium Hydride in the interstellar space was Tthe early universe was so hot and only had a few always a dilemma for astronomy for decades. types of atoms, mostly Lithium, Helium and Hydrogen. The During the 1970s, while examining the planetary nebula big bang theory explains the birth of the universe. In simple NGC-7027 scientists thought the environmental conditions, words, the universe evolved from the single molecules ultraviolet radiation and the heat of the ageing star might which then transformed for billions of years, becoming a be just apt for the formation of Helium Hydride. But these complex place. observations were inconclusive because of the lack of Molecular hydrogen is the constituent gas from evidence. The space telescopes used to detect the signal of which stars, galaxies and other complex structures in the Helium Hydride did not have the technology to pick out universe emerged. It is also the most abundant gas in the the specific signal from the pool of other molecules in the universe. Scientists believe that the helium and hydrogen nebula. began to combine to form the universe’s first molecule However, NASA’s Stratospheric Observatory for called Helium Hydride (HeH+). -
Panpsychism As an Observational Science
City University of New York (CUNY) CUNY Academic Works Publications and Research New York City College of Technology 2020 Panpsychism as an Observational Science Gregory Matloff CUNY New York City College of Technology How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/ny_pubs/636 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Journal of Consciousness Exploration & Research | August 2020 | Volume 11 | Issue 5 | pp. 468-486 468 Matloff, G. L., Panpsychism as an Observational Science Article Panpsychism as an Observational Science Gregory L. Matloff * Physics Dept., New York City College of Technology, CUNY, Brooklyn, NY, USA Abstract The metaphysical concept of panpsychism defines a field of proto-consciousness that is present at all levels in the universe. An observational indication that this concept might be correct is self- organization on all levels from the molecular to the galactic. In 2011, an investigation into the validity of author Olaf Stapledon’s concept that a portion of stellar motion is volitional led to a consideration of an observational stellar kinematics anomaly dubbed Parenago’s Discontinuity. Data available at the time indicated that cooler, less massive stars (such as the Sun) revolve a bit faster around the center of the Milky Way galaxy than hotter, more massive stars, at least in a sphere with a radius of ~260 light years centered on the Sun. The spectral signature of this discontinuity becomes evident at about the point in the stellar population where molecules can form in stellar photospheres, which supports a published model of molecular consciousness.