Characteristics of Macrophytes in the Lubigi Wetland in Uganda
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Impact of Climate Change at Lake Victoria
East Africa Living Lakes Network, C/O OSIENALA (Friends of Lake Victoria). Dunga Beach Kisumu Ann Nabangala Obae. Coordinator. Phone: +254-20-3588681. Email: [email protected] Background It is the Second largest fresh water lake in the World and it is surrounded by three East African States: With 6% in Kenya; Tanzania 52% and Uganda 42%. It is Located at 0:21 0 North and 3:00 0 South of the Equator Lake Victoria has a total length of 3,440 kms and 240 kms wide from East to West and is 1,134 meters above sea level with maximum depth of 82m.Its surface area is 68,870 km 2, catchment area of 180,950 km 2 Generally shallow with maximum depth of 84 meters and mean depth of 40 meters Average inflows and out flows of Lake Victoria Type of flow Flow (m3/s) Percentage (%) Inflows Rain over Lake 3,631 82 Basin Discharge 778 18 Type of flow Flow ( m3/s) Percentage (%) Out flows Evaporation from lake -3,300 76 Nile River - 1,046 24 Balance +33 Sources of Lake Victoria from Kenyan water towers Sondu Miriu river Yala river Nzoia river Mara River Kuja river Impacts and effects Changes in water budget are respectively accompanied by water level fluctuation and promote thermal structures which result in nutrient and food web dynamics. Studies have proved that there is a positive correlation between water level and fish landings (Williams, l972) The abundant fish catches are highly correlated with rainfall and lake levels. The records show that the catches reduced to between 60% and 70% during the current reduction of water level in Nyanza Gulf. -

Kyankwanzi Survey Report 2017
GROUND SURVEY FOR MEDIUM - LARGE MAMMALS IN KYANKWANZI CONCESSION AREA Report by F. E. Kisame, F. Wanyama, G. Basuta, I. Bwire and A. Rwetsiba, ECOLOGICAL MONITORING AND RESEARCH UNIT UGANDA WILDLIFE AUTHORITY 2018 1 | P a g e Contents Summary.........................................................................................................................4 1.0. INTRODUCTION ..................................................................................................5 1.1. Survey Objectives.....................................................................................................6 2.0. DESCRIPTION OF THE SURVEY AREA ..........................................................6 2.2. Location and Size .....................................................................................................7 2.2. Climate.....................................................................................................................7 2.3 Relief and Vegetation ................................................................................................8 3.0. METHOD AND MATERIALS..............................................................................9 Plate 1. Team leader and GPS person recording observations in the field.........................9 3.1. Survey design .........................................................................................................10 4.0. RESULTS .............................................................................................................10 4.1. Fauna......................................................................................................................10 -

Population, Distribution and Conservation Status of Sitatunga (Tragelaphus Spekei) (Sclater) in Selected Wetlands in Uganda
POPULATION, DISTRIBUTION AND CONSERVATION STATUS OF SITATUNGA (TRAGELAPHUS SPEKEI) (SCLATER) IN SELECTED WETLANDS IN UGANDA Biological -Life history Biological -Ecologicl… Protection -Regulation of… 5 Biological -Dispersal Protection -Effectiveness… 4 Biological -Human tolerance Protection -proportion… 3 Status -National Distribtuion Incentive - habitat… 2 Status -National Abundance Incentive - species… 1 Status -National… Incentive - Effect of harvest 0 Status -National… Monitoring - confidence in… Status -National Major… Monitoring - methods used… Harvest Management -… Control -Confidence in… Harvest Management -… Control - Open access… Harvest Management -… Control of Harvest-in… Harvest Management -Aim… Control of Harvest-in… Harvest Management -… Control of Harvest-in… Tragelaphus spekii (sitatunga) NonSubmitted Detrimental to Findings (NDF) Research and Monitoring Unit Uganda Wildlife Authority (UWA) Plot 7 Kira Road Kamwokya, P.O. Box 3530 Kampala Uganda Email/Web - [email protected]/ www.ugandawildlife.org Prepared By Dr. Edward Andama (PhD) Lead consultant Busitema University, P. O. Box 236, Tororo Uganda Telephone: 0772464279 or 0704281806 E-mail: [email protected] [email protected], [email protected] Final Report i January 2019 Contents ACRONYMS, ABBREVIATIONS, AND GLOSSARY .......................................................... vii EXECUTIVE SUMMARY ....................................................................................................... viii 1.1Background ........................................................................................................................... -

World Bank Document
Document of The World Bank Public Disclosure Authorized Report No: ICR00002916 IMPLEMENTATION COMPLETION AND RESULTS REPORT (IDA-43670) ON A CREDIT Public Disclosure Authorized IN THE AMOUNT OF SDR 22.0 MILLION (US$ 33.6 MILLION EQUIVALENT) TO THE REPUBLIC OF UGANDA FOR A KAMPALA INSTITUTIONAL AND INFRASTRUCTURE DEVELOPMENT ADAPTABLE PROGRAM LOAN (APL) PROJECT Public Disclosure Authorized June 27, 2014 Public Disclosure Authorized Urban Development & Services Practice 1 (AFTU1) Country Department AFCE1 Africa Region CURRENCY EQUIVALENTS (Exchange Rate Effective July 31, 2007) Currency Unit = Uganda Shillings (Ushs) Ushs 1.00 = US$ 0.0005 US$ 1.53 = SDR 1 FISCAL YEAR July 1 – June 30 ABBREVIATIONS AND ACRONYMS APL Adaptable Program Loan CAS Country Assistance Strategy CRCS Citizens Report Card Surveys CSOs Civil Society Organizations EA Environmental Analysis EIRR Economic Internal Rate of Return EMP Environment Management Plan FA Financing Agreement FRAP Financial recovery action plan GAAP Governance Assessment and Action Plan GAC Governance and Anti-corruption GoU Government of Uganda HDM-4 Highway Development and Management Model HR Human Resource ICR Implementation Completion Report IDA International Development Association IPF Investment Project Financing IPPS Integrated Personnel and Payroll System ISM Implementation Support Missions ISR Implementation Supervision Report KCC Kampala City Council KCCA Kampala Capital City Authority KDMP Kampala Drainage Master Plan KIIDP Kampala Institutional and Infrastructure Development Project -

EXAMING POOR DRAINAGE in BWAISE II PARISH, KAWEMPE DIVISION, KAMPALA the Streams in Bwaise II Parish Are No Longer in Their Natural State
2011 Mubiru Katya Paul Written by Sarah Namulondo EXAMING POOR DRAINAGE IN BWAISE II PARISH, KAWEMPE DIVISION, KAMPALA The streams in Bwaise II Parish are no longer in their natural state. They flow in straight courses and carry polluted water. This is because of the various forms of the both direct and indirect human interferences. Changes on the land surface consequent to urbanization constitute an indirect interference. EXAMINING POOR DRAINAGE IN BWAISE II PARISH, KAWEMPE DIVISION, KAMPALA BY MUBIRU KATYA PAUL A DISSERTATION SUBMITTED TO THE POSTGRADUATE SCHOOL AS PARTIAL FULFILLMENT FOR THE REQUIREMENTS FOR AWARD OF MASTERS DEGREE IN NATURAL RESOURCES MANAGEMENT OF NKUMBA UNIVERSITY SEPTEMBER 2011 2 DECLARATION I, MUBIRU KATYA PAUL, hereby declare that this dissertation is my original work and has never been published or submitted to any university or institution of higher learning for the award of a Post graduate Degree. SIGNED……………………………………………………………………………….. DATE…………………………………………………………………………………… i APPROVAL This dissertation has been produced under my supervision and has been submitted with my approval as the university supervisor for examination and award of Masters of Science degree in Natural Resources Management. Professor Eric Edroma ………………. ..................... SUPERVISOR Signed Date ii DEDICATION This dissertation is dedicated to: I. The people who will find my research data useful in helping to implement and cover the gaps of Environmental law, II. The members of my family who have supported me emotionally and financially throughout my academic career, III. My mother and father Mr and Mrs Katya II Difasi (Senior of Nawansenga Iganga District) and my Aunt Hanifa Nadongo Walangira (Bulebi- Bugiri) who sacrificed a lot for my education, and IV. -

The Charcoal Grey Market in Kenya, Uganda and South Sudan (2021)
COMMODITY REPORT BLACK GOLD The charcoal grey market in Kenya, Uganda and South Sudan SIMONE HAYSOM I MICHAEL McLAGGAN JULIUS KAKA I LUCY MODI I KEN OPALA MARCH 2021 BLACK GOLD The charcoal grey market in Kenya, Uganda and South Sudan ww Simone Haysom I Michael McLaggan Julius Kaka I Lucy Modi I Ken Opala March 2021 ACKNOWLEDGEMENTS The authors would like to thank everyone who gave their time to be interviewed for this study. They would like to extend particular thanks to Dr Catherine Nabukalu, at the University of Pennsylvania, and Bryan Adkins, at UNEP, for playing an invaluable role in correcting our misperceptions and deepening our analysis. We would also like to thank Nhial Tiitmamer, at the Sudd Institute, for providing us with additional interviews and information from South Sudan at short notice. Finally, we thank Alex Goodwin for excel- lent editing. Interviews were conducted in South Sudan, Uganda and Kenya between February 2020 and November 2020. ABOUT THE AUTHORS Simone Haysom is a senior analyst at the Global Initiative Against Transnational Organized Crime (GI-TOC), with expertise in urban development, corruption and organized crime, and over a decade of experience conducting qualitative fieldwork in challenging environments. She is currently an associate of the Oceanic Humanities for the Global South research project based at the University of the Witwatersrand in Johannesburg. Ken Opala is the GI-TOC analyst for Kenya. He previously worked at Nation Media Group as deputy investigative editor and as editor-in-chief at the Nairobi Law Monthly. He has won several journalistic awards in his career. -

———— “Mudo”: the Soga 'Little Red Riding Hood'
LILLIAN BUKAAYI TIBASIIMA ———— º “Mudo”: The Soga ‘Little Red Riding Hood’ ABSTRACT This essay analyses the social underpinnings of the oral tale of “Mudo,” which belongs to the Aarne–Thompson tale type 333, along with a group of similar tales that resemble the action and movement of “Little Red Riding Hood.” Basic to the exposition is Adolf Bastian’s assertion of the fundamental similarity of ideas between all social groups. In the “Mudo” story and its Ugandan variants, the victim is a solitary little girl and the villain a male ogre who devises ways of eating her; the ogre is mostly successful, although in some variants the girl manages to escape. Although these tales come from a great range of cultures and different geographical locations, and the counterpart of the ogre in the European tales is a wolf in disguise, they share elements of plot, characteriza- tion, and motif, and address similar concerns. Introduction USOGA IS PART OF EAS TERN UGANDA, surrounded by water. The B Rev. Fredrick Kisuule Kaliisa1 notes: To the west is river Kiira (Nile) marking the boundary between Buganda and Busoga. To the East is river Mpologoma separating Busoga from Bukedi. To the North are river Mpologoma and Lake Kyoga, forming the boundary be- tween Busoga and Lango. To the south, is Lake Victoria (Nalubaale). It might be the result of the geographical location of Busoga that ogre stories were composed to warn the people against impending harm if they went out alone and stayed in secluded places. Nnalongo Lukude emphasizes this: Historically, Busoga was surrounded by bodies of water and forests, it was very bushy and as a result harboured many wild animals, some of which were man-eaters. -
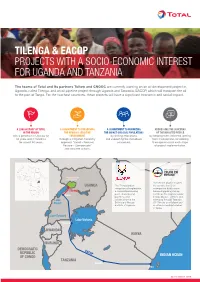
Tilenga & Eacop Projects with a Socio-Economic Interest for Uganda
TILENGA & EACOP PROJECTS WITH A SOCIO-ECONOMIC INTEREST FOR UGANDA AND TANZANIA The teams of Total and its partners Tullow and CNOOC are currently working on an oil development project in Uganda, called Tilenga, and an oil pipeline project through Uganda and Tanzania, EACOP, which will transport the oil to the port of Tanga. For the two host countries, these projects will have a significant economic and social impact. A LONG HISTORY OF TOTAL A COMMITMENT TO PRESERVING A COMMITMENT TO MINIMIZING ADDRESSING THE CONCERNS IN THE REGION THE REGION'S SENSITIVE THE IMPACT ON LOCAL POPULATIONS OF THE IMPACTED PEOPLE with a presence in Uganda for ENVIRONMENT by limiting relocations by keeping them informed, getting 60 years and in Tanzania through a mitigation hierarchy and supporting the individuals them involved and considering for almost 50 years. approach “Avoid – Reduce/ concerned. their opinions into each stage Restore – Compensate” of project implementation. and concrete actions. SOUDAN DU SUD ÉTHIOPIE Murchison Falls National Park The EACOP project involves UGANDA The Tilenga project the construction of an comprises oil exploration, underground hydrocarbon Tilenga a crude oil processing transport pipeline starting Hoima plant, underground just inside the Uganda border pipelines, and (Hoima District - 297km) and Lake infrastructure in the extending through Tanzania Albert Buliisa and Nwoya (1147km) to an oil depot and districts of Uganda. an offshore loading terminal in Tanga. Lake Edward Lake Victoria Bukoba RWANDA KENYA BURUNDI DEMOCRATIC EACOP REPUBLIC INDIAN OCEAN OF CONGO TANZANIA Singida Tanga SEPTEMBER 2019 ZAMBIE MOZAMBIQUE MALAWI SOUDAN DU SUD THIOPIE FOCUS ON THE TILENGA PROJECT Total E&P Uganda, fully aware of the project's sensitive nature, has placed particular emphasis on environmental and societal issues, with a specific commitment to leaving the site in a better state than it was before the work started and to limiting residents' relocations as much as possible. -

Impact Evaluation: Lake Victoria Transport Corridor Project
RWANDA Impact Evaluation: Lake Victoria Transport Corridor Project What is the impact of the highway construction on travel costs and aggregate economic activity? Context Lake Victoria Transport Despite recent growth, Rwanda remains amongst the poorest Program — SOP1, Rwanda countries in the world. As a landlocked country, high transport IMPLEMENTING AGENCY: costs are a critical constraint to growth. According to the Business Rwanda Transport Development Environment and Enterprise Performance (BEEP) 2011 report, about Agency (RTDA) 60 percent of firms in Rwanda rely on imports for inputs and/or supplies, which take an average of 15 days to clear customs. INTERVENTION: Construction of 119km of new, high- The Government of Rwanda (GoR) has assigned fundamental capacity highway in the Eastern and importance to the development of the economic infrastructure of Southern provinces, replacing the the country, and particularly to road transportation. The construction existing gravel road and providing of the Ngoma-Nyanza highway is identified as a priority investment new connectivity to the Tanzania port for the government under the most recent poverty reduction of entry. strategy. As depicted in blue in Figure 1, this new highway consists of two separate segments that will replace the existing gravel road PROJECT DEVELOPMENT OBJECTIVE and will provide new connectivity within the National Road System To contribute to the efficient and between the Tanzania port of entry in the south east of the country safe movement of goods and people and southern central Rwanda. The first section has a length of along the regional corridor from the border crossing at Rusumo to the border crossing at Nemba. -

The Precolonial Social Formation Among the Bakenhe Fishing Community
The Precolonial Social Formation Among the Bakenhe Fishing Community .. of Lake Kyoga Il.egion of Uganda , 1800- 1894* • By C. Asowa- Okwe , Department of Political Science and Public Adminis t rat i on. Int roduction It i s eminently evident from the literqture ava ilable on the pre- colonia l history of Uganda and East Africa in general , that one area which has either been negl ected , or peripher ally treat ed i s that of the fishing industry and the fishing communities . A car eful and close examina tion of these liter a ture show a definite bia s towar ds the agricult ural and pastoral communities and their economic activities. And as if tha t is not enough , f ew that have t r i ed to grappl e with the fishing industry have l ar gely tended to be descriptive/narra tive , and ma inly, t alking a bout methods of fishing and the types of fis hes c aught. In f act the bulk of literature ~ on the fishing industry are basically , works of the physical scientists who ar e mainl y tra i ned in bi ol ogica l sciences . These studies , ther ef or e , ma i nly focus on the fish species f ound in the wa t ers of East African l akes , rivers , ponds and swamps , their f ood r equirements and dist ribut ion. They a lso concern thems elves with the question of density of fish popu- l a tion, the growth r a t e of i ndividua l species, the age at which they mature , t he specific f actor s which cause ornt~o p ic a l l ake to support many fish and another r el a tively f ew, the depletion of certa in fish species , the stocking of new species, and how t o check .the depl etion of s ome species, like·, ales.tes ( soga) , Labeo (ningu), bagrus (semutundu) . -

MY LORD and MY GOD! (Jn
MY LORD AND MY GOD! (Jn. 20:28) By Fr. Dr. Deogratias Ssonko Dedication TO MOTHER MARY, Our model of Faith: ”Blessed are you who believed that what was spoken to you by the Lord would be fulfilled” Lk.1:45 And TO SAINT THOMAS THE APOSTLE Model of Doubters: “My Lord and My God” Jn. 20:28 Table of Contents PART ONE: The Teaching) Ministry Chapter I: Creation and Early Formation The mystery of Creation……………………………………………………. Each Person is Unique ……………………………………………………… My very identity…………………………………………………………….. God “Teaches” in all Activities…………………………………………….. My “Constantly Learning” Brothers and Sisters…………………………... My “Prophetic Teaching” Parents………………………………………….. Studying at a Protestant School…………………………………………….. Two great Masters Prior to My Seminary Life…………………………….. A Head Teacher who trained us the Hard way……………………………. Chapter II: Seminary Formation My Seminary Alma Mater(s) ……………………………………………… Spiritual Life Formation…………………………………………………… Seminary Discipline……………………………………………………….. Academic Life……………………………………………………………... Liturgy and Music Formation……………………………………………... Extra Curricular Activities ………………………………………………... Team Work Formation ……………………………………………………. Holidays …………………………………………………………………… My All in All “Silver” Experience in the Teaching Ministry …………….. PART TWO: The Sanctifying Ministry Chapter III: Priesthood and Sanctity Liturgy as a Cerebration of my very Identity ……………………………. Soccer Opens my Heart to the Priesthood ………………………………. The Curious Mass Server …………….. ………………………………… My first Steps to the Holy Orders ………………………………………. -

The Fishesof Uganda-I
1'0 of the Pare (tagu vaIley.': __ THE FISHES OF UGANDA-I uku-BujukUf , high peaks' By P. H. GREENWOOD Fons Nilus'" East African Fisheries Research Organization ~xplorersof' . ;ton, Fresh_ CHAPTER I I\.bruzzi,Dr: knowledge : INTRODUCTION ~ss to it, the ,THE fishes of Uganda have been subject to considerable study. Apart from .h to take it many purely descriptive studies of the fishes themselves, three reports have . been published which deal with the ecology of the lakes in relation to fish and , fisheries (Worthington (1929a, 1932b): Graham (1929)).Much of the literature is scattered in various scientific journals, dating back to the early part of the ; century and is difficult to obtain iIi Uganda. The more recent reports also are out of print and virtually unobtainable. The purpose .of this present survey is to bring together the results of these many researches and to present, in the light of recent unpublished information, an account of the taxonomy and biology of the many fish species which are to be found in the lakes and rivers of Uganda. Particular attention has been paid to the provision of keys, so that most of the fishesmay be easily identified. It is hardly necessary to emphasize that our knowledge of the East African freshwater fishes is still in an early and exploratory stage of development. Much that has been written is known to be over-generalized, as conclusions were inevitably drawn from few and scattered observations or specimens. From the outset it must be stressed that the sections of this paper dealing with the classification and description of the fishes are in no sense a full tax- onomicrevision although many of the descriptions are based on larger samples than were previously available.