ANNUAL WORK PLAN Act to End Ntds | West Program Cote D'ivoire
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Côte D'ivoire
CÔTE D’IVOIRE COI Compilation August 2017 United Nations High Commissioner for Refugees Regional Representation for West Africa - RSD Unit UNHCR Côte d’Ivoire UNHCR Regional Representation for West Africa - RSD Unit UNHCR Côte d’Ivoire Côte d’Ivoire COI Compilation August 2017 This report collates country of origin information (COI) on Côte d’Ivoire up to 15 August 2017 on issues of relevance in refugee status determination for Ivorian nationals. The report is based on publicly available information, studies and commentaries. It is illustrative, but is neither exhaustive of information available in the public domain nor intended to be a general report on human-rights conditions. The report is not conclusive as to the merits of any individual refugee claim. All sources are cited and fully referenced. Users should refer to the full text of documents cited and assess the credibility, relevance and timeliness of source material with reference to the specific research concerns arising from individual applications. UNHCR Regional Representation for West Africa Immeuble FAALO Almadies, Route du King Fahd Palace Dakar, Senegal - BP 3125 Phone: +221 33 867 62 07 Kora.unhcr.org - www.unhcr.org Table of Contents List of Abbreviations .............................................................................................................. 4 1 General Information ....................................................................................................... 5 1.1 Historical background ............................................................................................ -

Agricultural Microcredit in Cote D'ivoire
DOTTORATO IN SCIENZE ECONOMICHE, AZIENDALI E STATISTICHE DIPARTIMENTO DI SCIENZE ECONOMICHE, AZIENDALI E STATISTICHE SECS-P/06 AGRICULTURAL MICROCREDIT IN COTE D’IVOIRE IL DOTTORE IL DECANO DEL COLLEGIO MOUSTAPHA SANGARE Chiar.mo Prof. FABIO MAZZOLA IL TUTOR Chiar.mo Prof. VINCENZO PROVENZANO CICLO XXIX ANNO CONSEGUIMENTO TITOLO 2017 ! ABSTRACT The financing of agriculture in rural areas for poverty reduction remains an important concern that has involved several research. The granting of microcredit to people with low income is viewed as an opportunity to increase farm income in rural areas. The survey led in Côte d’Ivoire in the region of Tchologo attempted to shed light on the relation between microfinance institution and farmers. This region has about 65% of poverty rate and people are mainly involved in the production of cash and food crops. Through financial diaries methodology, farm needs were tracked to know how farmers manage his little financial resources by the identification of main farm needs and their difficulties to meet them. Indeed, the survey takes into account 202 respondents with farm as main profile among which 34% are credit beneficiaries of UNACOOPEC-Ferké institution. The analysis of the economic and social characteristics provides various information regarding the consideration that grants microfinance institution to farmers. Besides that, the statistical significance (t-test) allowed to dress the concern of the improving of farm income through microcredit supplying. ! ACRONYM ANADER Agence Nationale d’Appui au -

Full Issue 117
South African Journal of Science volume 117 number 1/2 High lightning risk for rural communities in SA Predicting take-up of home loans using tree-based ensemble models Monitoring and conservation of terrestrial and marine ecosystems in SA Hominin lower limb bones from Sterkfontein Caves HIV self-testing is user-friendly and accurate Volume 117 Number 1/2 January/February 2021 EDITOR-IN-CHIEF Jane Carruthers Academy of Science of South Africa MANAGING EDITOR Linda Fick Academy of Science of South Africa ONLINE PUBLISHING South African SYSTEMS ADMINISTRATOR Nadia Grobler Journal of Science Academy of Science of South Africa ASSOCIATE EDITORS Margaret Avery Cenozoic Studies, Iziko Museums of South Africa, South Africa Priscilla Baker eISSN: 1996-7489 Department of Chemistry, University of the Western Cape, South Africa Pascal Bessong HIV/AIDS & Global Health Research Leader Programme, University of Venda, South Africa Celebrating multidisciplinarity Jennifer Case Jane Carruthers ....................................................................................................................... 1 Department of Engineering Education, Virginia Tech, Blacksburg, VA, USA Book Reviews Teresa Coutinho Department of Microbiology and Invasion science in South Africa: The definitive collection Plant Pathology, University of Pretoria, South Africa Philip E. Hulme ........................................................................................................................ 2 Tania Douglas The University of Cape Town: Between apartheid -

Côte D'ivoire Country Focus
European Asylum Support Office Côte d’Ivoire Country Focus Country of Origin Information Report June 2019 SUPPORT IS OUR MISSION European Asylum Support Office Côte d’Ivoire Country Focus Country of Origin Information Report June 2019 More information on the European Union is available on the Internet (http://europa.eu). ISBN: 978-92-9476-993-0 doi: 10.2847/055205 © European Asylum Support Office (EASO) 2019 Reproduction is authorised, provided the source is acknowledged, unless otherwise stated. For third-party materials reproduced in this publication, reference is made to the copyrights statements of the respective third parties. Cover photo: © Mariam Dembélé, Abidjan (December 2016) CÔTE D’IVOIRE: COUNTRY FOCUS - EASO COUNTRY OF ORIGIN INFORMATION REPORT — 3 Acknowledgements EASO acknowledges as the co-drafters of this report: Italy, Ministry of the Interior, National Commission for the Right of Asylum, International and EU Affairs, COI unit Switzerland, State Secretariat for Migration (SEM), Division Analysis The following departments reviewed this report, together with EASO: France, Office Français de Protection des Réfugiés et Apatrides (OFPRA), Division de l'Information, de la Documentation et des Recherches (DIDR) Norway, Landinfo The Netherlands, Immigration and Naturalisation Service, Office for Country of Origin Information and Language Analysis (OCILA) Dr Marie Miran-Guyon, Lecturer at the École des Hautes Études en Sciences Sociales (EHESS), researcher, and author of numerous publications on the country reviewed this report. It must be noted that the review carried out by the mentioned departments, experts or organisations contributes to the overall quality of the report, but does not necessarily imply their formal endorsement of the final report, which is the full responsibility of EASO. -

Assessment of the Implementation of Alternative Process Technologies for Rural Heat and Power Production from Cocoa Pod Husks
Assessment of the implementation of alternative process technologies for rural heat and power production from cocoa pod husks Dimitra Maleka Master of Science Thesis KTH School of Industrial Engineering and Management Department of Energy Technology Division of Heat and Power Technology SE-100 44 STOCKHOLM Master of Science Thesis EGI 2016: 034 MSC EKV1137 Assessment of the implementation of alternative process technologies for rural heat and power production from cocoa pod husks Dimitra Maleka Approved Examiner Supervisors Reza Fakhraie Reza Fakhraie (KTH) David Bauner (Renetech AB) Commissioner Contact person ii Abstract Cocoa pod husks are generated in Côte d’Ivoire, in abundant quantities annually. The majority is left as waste to decompose at the plantations. A review of the ultimate and proximate composition of CPH resulted in the conclusion that, CPH is a high potential feedstock for both thermochemical and biochemical processes. The main focus of the study was the utilization of CPH in 10,000 tons/year power plants for generation of energy and value-added by-products. For this purpose, the feasibility of five energy conversion processes (direct combustion, gasification, pyrolysis, anaerobic digestion and hydrothermal carbonization) with CPH as feedstock, were investigated. Several indicators were used for the review and comparison of the technologies. Anaerobic digestion and hydrothermal carbonization were found to be the most suitable conversion processes. For both technologies an analysis was conducted including technical, economic, environmental and social aspects. Based on the characterization of CPH, appropriate reactors and operating conditions were chosen for the two processes. Moreover, the plants were chosen to be coupled with CHP units, for heat and power generation. -

Interrupting Seasonal Transmission of Schistosoma Haematobium and Control of Soil-Transmitted Helminthiasis in Northern and Cent
Tian-Bi et al. BMC Public Health (2018) 18:186 DOI 10.1186/s12889-018-5044-2 STUDYPROTOCOL Open Access Interrupting seasonal transmission of Schistosoma haematobium and control of soil-transmitted helminthiasis in northern and central Côte d’Ivoire: a SCORE study protocol Yves-Nathan T. Tian-Bi1,2*, Mamadou Ouattara1,2, Stefanie Knopp3,4, Jean T. Coulibaly1,2,3,4, Eveline Hürlimann3,4, Bonnie Webster5, Fiona Allan5, David Rollinson5, Aboulaye Meïté6, Nana R. Diakité1,2, Cyrille K. Konan1,2, Eliézer K. N’Goran1,2 and Jürg Utzinger3,4* Abstract Background: To achieve a world free of schistosomiasis, the objective is to scale up control and elimination efforts in all endemic countries. Where interruption of transmission is considered feasible, countries are encouraged to implement a comprehensive intervention package, including preventive chemotherapy, information, education and communication (IEC), water, sanitation and hygiene (WASH), and snail control. In northern and central Côte d’Ivoire, transmission of Schistosoma haematobium is seasonal and elimination might be achieved. In a cluster-randomised trial, we will assess different treatment schemes to interrupt S. haematobium transmission and control soil-transmitted helminthiasis over a 3-year period. We will compare the impact of (i) arm A: annual mass drug administration (MDA) with praziquantel and albendazole before the peak schistosomiasis transmission season; (ii) arm B: annual MDA after the peak schistosomiasis transmission season; (iii) arm C: two yearly treatments before and after peak schistosomiasis transmission; and (iv) arm D: annual MDA before peak schistosomiasis transmission, coupled with chemical snail control using niclosamide. Methods/design: The prevalence and intensity of S. -
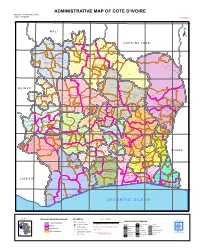
ADMINISTRATIVE MAP of COTE D'ivoire Map Nº: 01-000-June-2005 COTE D'ivoire 2Nd Edition
ADMINISTRATIVE MAP OF COTE D'IVOIRE Map Nº: 01-000-June-2005 COTE D'IVOIRE 2nd Edition 8°0'0"W 7°0'0"W 6°0'0"W 5°0'0"W 4°0'0"W 3°0'0"W 11°0'0"N 11°0'0"N M A L I Papara Débété ! !. Zanasso ! Diamankani ! TENGRELA [! ± San Koronani Kimbirila-Nord ! Toumoukoro Kanakono ! ! ! ! ! !. Ouelli Lomara Ouamélhoro Bolona ! ! Mahandiana-Sokourani Tienko ! ! B U R K I N A F A S O !. Kouban Bougou ! Blésségué ! Sokoro ! Niéllé Tahara Tiogo !. ! ! Katogo Mahalé ! ! ! Solognougo Ouara Diawala Tienny ! Tiorotiérié ! ! !. Kaouara Sananférédougou ! ! Sanhala Sandrégué Nambingué Goulia ! ! ! 10°0'0"N Tindara Minigan !. ! Kaloa !. ! M'Bengué N'dénou !. ! Ouangolodougou 10°0'0"N !. ! Tounvré Baya Fengolo ! ! Poungbé !. Kouto ! Samantiguila Kaniasso Monogo Nakélé ! ! Mamougoula ! !. !. ! Manadoun Kouroumba !.Gbon !.Kasséré Katiali ! ! ! !. Banankoro ! Landiougou Pitiengomon Doropo Dabadougou-Mafélé !. Kolia ! Tougbo Gogo ! Kimbirila Sud Nambonkaha ! ! ! ! Dembasso ! Tiasso DENGUELE REGION ! Samango ! SAVANES REGION ! ! Danoa Ngoloblasso Fononvogo ! Siansoba Taoura ! SODEFEL Varalé ! Nganon ! ! ! Madiani Niofouin Niofouin Gbéléban !. !. Village A Nyamoin !. Dabadougou Sinémentiali ! FERKESSEDOUGOU Téhini ! ! Koni ! Lafokpokaha !. Angai Tiémé ! ! [! Ouango-Fitini ! Lataha !. Village B ! !. Bodonon ! ! Seydougou ODIENNE BOUNDIALI Ponondougou Nangakaha ! ! Sokoro 1 Kokoun [! ! ! M'bengué-Bougou !. ! Séguétiélé ! Nangoukaha Balékaha /" Siempurgo ! ! Village C !. ! ! Koumbala Lingoho ! Bouko Koumbolokoro Nazinékaha Kounzié ! ! KORHOGO Nongotiénékaha Togoniéré ! Sirana -

Côte D'ivoire
AFRICAN DEVELOPMENT BANK GROUP PROJECT: ROAD DEVELOPMENT AND TRANSPORT FACILITATION PROGRAMME WITHIN THE MANO RIVER UNION COUNTRY: COTE D’IVOIRE, GUINEA, AND LIBERIA SUMMARY ENVIRONMENTAL AND SOCIAL IMPACT ASSESSMENT (ESIA) Team Leader J. N. ILBOUDO, Transport Engineer OITC.1 5012 J.B. AGUMA, Transport Economist OITC.1 1956 J. P.M. KALALA , Chief Socio-Economist OITC.1 3561 N. KULEMEKA, Chief Socio-Economist SARC/ONEC.3 8452 L. M. KINANE, Environmentalist ONEC.3 2933 E. NDINYA, Environmentalist SARC/ONEC.3 1541 LRFO/OITC.1 7072 P. TAMBAH, Infrastructure Engineer Team Members Project Malick Soumare, Procurement Specialist ORPF.1/ LRFO Team M. H . SANON , Socio-Economist ONEC-3 Mose Mabe-Koofhethile, Procurement Specialist ORPF.1/ SNFO Sector Division Jean Kizito KABANGUKA OITC1 2143 Manager Sector Director Amadou OUMAROU OITC 3075 Regional Director Franck Joseph PERRAULT ORWA 4046 Director, Regional Janvier K. LITSE ONRI 4047 Integration and Trade 2 SUMMARY ENVIRONMENTAL AND SOCIAL IMPACT ASSESSMENT Project Name : Road Development and Transport Project No.: P-Z1-DB0-103 Facilitation Programme Within the Mano River Union Countries: Cote d’Ivoire, Guinea and Liberia Depart ment : OITC Division: OITC-1 Introduction This document is a Summary Environmental and Social Impact Assessment (ESIA) of the Ivorian section of the Road Development and Transport Facilitation Programme within the Mano River Union. The summary has been prepared in accordance with the guidelines and procedures for environmental and social assessment of the African Development Bank (AfDB) for Category 1 projects, as well as with the policies in force in Côte d'Ivoire. The project description and rationale are presented first, followed by the applicable legal and institutional framework in Côte d'Ivoire. -

OPS) for SRP’S / Flash Appeals
Online Planning / Project System (OPS) for SRP’s / Flash Appeals CHAP Section October 2013 http://ops.unocha.org/ OPS Topics and learning objectives What is OPS? How to register in OPS? How to upload a project? How to use OPS data? What is OPS? Who can access it? What is it for? Complete the registration process online. Click on New User to start the process Create your account (indicate your e-mail, set your password) Complete your User Profile online (contact details, select your “role”, select your organization) Submit the access request to the OPS administrator Check your e-mail! The OPS Administrator will send you the access link within 24h Activate the access link received by clicking on it •Create or revise projects of your own organisation and review other projects. Click on the PDF/WORD icons at the top of the project online form Download the full project sheet Who can do what when Field Process HQs process Roles Draft Approved Agencies HC Final by cluster HQ Review UN/NGO Field Edit View View View Programme Officer Cluster Lead Edit Edit View View HC Edit Edit View Edit OCHA Field Edit Edit View View Agencies/NGO Edit View Edit View HQs OCHA HQ View View View Edit OCHA CAP Edit Edit Edit Edit THE GENDER MARKER Using the OPS data Review projects and process Contact lists Who is doing what where: spot gaps and overlaps tables OPS and FTS Projects list with project sheets FTS list of projects and project sheets OPS and FTS Appeal document Projects volume For further information: Financial Tracking Service (FTS): -

Ivory Coast Strategy Action Plan
International Rescue Committee Côte d’Ivoire: Strategy Action Plan A Wade A IRC / Revised June 2019 JSoha / IRC / IRC2020 GLOBAL STRATEGY OVERVIEW The International Rescue Committee’s (IRC) mission is to help the world’s most vulnerable people survive, recover, and gain control of their future. The aim of the global strategy, IRC2020 (see right), is to make measurable improvements in health, safety, education, economic wellbeing, and decision-making power. Therefore, the IRC will make investments to have more effective programs, use resources more efficiently, reach more people more quickly and better respond to beneficiaries’ needs. THE IRC IN CÔTE D’IVOIRE: STRATEGY ACTION PLAN 2 CÔTE D’IVOIRE OVERVIEW Civil war in 2002 left Côte d’Ivoire in a fragile socio- mismanagement often forces patients to pay for economic situation, which was further compounded services to which they are entitled. These financial by the 2010-2011 post-election crisis. Today, social barriers to services disproportionately impact services are often of poor quality and limited women and children, who usually do not have availability, which augments growing inequality and control over household expenditures. jeopardizes the country’s return to long term stability. As a result, a large part of the population is uneducated, in poor health, vulnerable to violence Despite strong economic growth in recent years, or exploitation, and with limited economic many populations are still at risk. Rural opportunities. communities and women and girls are often deprived of healthcare and education, and have a The IRC’s strategy for Côte d’Ivoire illustrates its lower quality of life. In addition, women and girls are commitment to improving the health, safety, highly vulnerable to gender-based violence. -

Cote D'ivoire Summary Full Resettlement Plan (Frp)
AFRICAN DEVELOPMENT BANK GROUP PROJECT : GRID REINFORCEMENT AND RURAL ELECTRIFICATION PROJECT COUNTRY : COTE D’IVOIRE SUMMARY FULL RESETTLEMENT PLAN (FRP) Project Team : Mr. R. KITANDALA, Electrical Engineer, ONEC.1 Mr. P. DJAIGBE, Principal Energy Officer ONEC.1/SNFO Mr. M.L. KINANE, Principal Environmental Specialist ONEC.3 Mr. S. BAIOD, Consulting Environmentalist, ONEC.3 Project Team Sector Director: Mr. A.RUGUMBA, Director, ONEC Regional Director: Mr. A. BERNOUSSI, Acting Director, ORWA Division Manager: Mr. Z. AMADOU, Division Manager, ONEC.1, 1 GRID REIFORCEMENT AND RURAL ELECTRIFICATION PROJECT Summary FRP Project Name : GRID REIFORCEMENT AND RURAL ELECTRIFICATION PROJECT Country : COTE D’IVOIRE Project Number : P-CI-FA0-014 Department : ONEC Division: ONEC 1 INTRODUCTION This document presents the summary Full Resettlement Plan (FRP) of the Grid Reinforcement and Rural Electrification Project. It defines the principles and terms of establishment of indemnification and compensation measures for project affected persons and draws up an estimated budget for its implementation. This plan has identified 543 assets that will be affected by the project, while indicating their socio- economic status, the value of the assets impacted, the terms of compensation, and the institutional responsibilities, with an indicative timetable for its implementation. This entails: (i) compensating owners of land and developed structures, carrying out agricultural or commercial activities, as well as bearing trees and graves, in the road right-of-way for loss of income, at the monetary value replacement cost; and (ii) encouraging, through public consultation, their participation in the plan’s planning and implementation. 1. PROJECT DESCRIPTION AND IMPACT AREA 1.1. Project Description and Rationale The Grid Reinforcement and Rural Electrification Project seeks to strengthen power transmission infrastructure with a view to completing the primary network, ensuring its sustainability and, at the same time, upgrading its available power and maintaining its balance. -

Côte D'ivoire
Côte d’Ivoire Monthly Humanitarian Report October 2011 www.unocha.org The mission of the United Nations Office for the Coordination of Humanitarian Affairs (OCHA) is to mobilize and coordinate effective and principled humanitarian action in partnership with national and international actors. Coordination Saves Lives • Celebrating 20 years of coordinated humanitarian action October 2011 Côte d’Ivoire Humanitarian Bulletin | 2 Côte d’Ivoire Monthly Humanitarian Report Coordination Saves Lives No.1 | October 2011 HIGHLIGHTS ■ The number of Internally Displaced People (IDPs) on 35 sites across the country is gradually decreasing. ■ A National Committee for Coordination of Humanitarian Action (CNCAH) was established by ministerial decree (Minister of State, Employment, Social Affairs and Solidarity) on 5 October 2011. ■ Ivorian refugees in Liberia are spontaneously and gradually returning to their villages of origin in the Moyen Cavally region. ■ The Humanitarian Coordinator and the Ivorian Minister for Employment, Solidarity and Social Affairs visited several European capitals to mobilize support for humanitarian action in Côte d'Ivoire. ■ The Special Representative of the UN Secretary General in Côte d'Ivoire took office on 24 October. I. GENERAL CONTEXT During October, violent incidents took place in the towns of Issia, Guiglo and Bangolo in the West of the country. These incidents resulted in the displacement of an estimated 450 people according to the Protection Cluster. In the Lagunes region, there has been an upsurge of security incidents: several cases of armed robbery, home intrusion and theft are reported to have been committed by armed men in the districts of Anyama, Abobo and Yopougon, in Abidjan. This situation is reportedly due to the free circulation of firearms and to escapee- prisoners since the post-electoral crisis.