The Effect of Climate on Population Dynamics of Butterfly Species Across Southeastern Virginia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Butterflies of the Wesleyan Campus
BUTTERFLIES OF THE WESLEYAN CAMPUS SWALLOWTAILS Hairstreaks (Subfamily - Theclinae) (Family PAPILIONIDAE) Great Purple Hairstreak - Atlides halesus Coral Hairstreak - Satyrium titus True Swallowtails Banded Hairstreak - Satyrium calanus (Subfamily - Papilioninae) Striped Hairstreak - Satyrium liparops Pipevine Swallowtail - Battus philenor Henry’s Elfin - Callophrys henrici Zebra Swallowtail - Eurytides marcellus Eastern Pine Elfin - Callophrys niphon Black Swallowtail - Papilio polyxenes Juniper Hairstreak - Callophrys gryneus Giant Swallowtail - Papilio cresphontes White M Hairstreak - Parrhasius m-album Eastern Tiger Swallowtail - Papilio glaucus Gray Hairstreak - Strymon melinus Spicebush Swallowtail - Papilio troilus Red-banded Hairstreak - Calycopis cecrops Palamedes Swallowtail - Papilio palamedes Blues (Subfamily - Polommatinae) Ceraunus Blue - Hemiargus ceraunus Eastern-Tailed Blue - Everes comyntas WHITES AND SULPHURS Spring Azure - Celastrina ladon (Family PIERIDAE) Whites (Subfamily - Pierinae) BRUSHFOOTS Cabbage White - Pieris rapae (Family NYMPHALIDAE) Falcate Orangetip - Anthocharis midea Snouts (Subfamily - Libytheinae) American Snout - Libytheana carinenta Sulphurs and Yellows (Subfamily - Coliadinae) Clouded Sulphur - Colias philodice Heliconians and Fritillaries Orange Sulphur - Colias eurytheme (Subfamily - Heliconiinae) Southern Dogface - Colias cesonia Gulf Fritillary - Agraulis vanillae Cloudless Sulphur - Phoebis sennae Zebra Heliconian - Heliconius charithonia Barred Yellow - Eurema daira Variegated Fritillary -

Impact of Non-Lethal Genetic Sampling on the Survival, Longevity and Behaviour of the Hermes Copper (Lycaena Hermes) Butterfly
Insect Conservation and Diversity (2013) doi: 10.1111/icad.12024 Impact of non-lethal genetic sampling on the survival, longevity and behaviour of the Hermes copper (Lycaena hermes) butterfly 1 2 3 DANIEL A. MARSCHALEK, JULIA A. JESU and MARK E. BERRES 1Department of Entomology, University of Wisconsin, Madison, WI, USA, 2Biology Department, San Diego State University, San Diego, CA, USA and 3Department of Animal Sciences, University of Wisconsin, Madison, WI, USA Abstract. 1. Genetic techniques are important tools for conservation, but tissue sampling for DNA analysis can be particularly detrimental to small study organisms. Historically, obtaining DNA samples from small insects and butter- flies has involved destructive (lethal) methods. 2. Recent improvements to DNA purification technologies have increased the likelihood that non-lethal sampling will be successful. In spite of this, only a few studies have evaluated the impacts of sampling on survival and behaviour. 3. The Hermes copper, Lycaena hermes (Edwards), butterfly has a restricted distribution and generally less than 10 individuals are encountered at any one location. Non-lethal DNA sampling would allow for genetic studies that have the potential to augment conservation decisions without causing local extirpations. 4. We demonstrate that removing a leg from an adult male Hermes copper does not have a measureable effect on their survival, longevity or behaviour. In addition, a single leg provides a sufficient DNA sample for amplified fragment length polymorphism studies. 5. The Hermes copper butterfly represents the smallest butterfly species for which the survival and behaviour has been assessed in relation to non-lethal tis- sue sampling. This suggests that research involving smaller and more delicate species could utilise leg removal as a non-lethal genetic sampling technique. -

Insect Survey of Four Longleaf Pine Preserves
A SURVEY OF THE MOTHS, BUTTERFLIES, AND GRASSHOPPERS OF FOUR NATURE CONSERVANCY PRESERVES IN SOUTHEASTERN NORTH CAROLINA Stephen P. Hall and Dale F. Schweitzer November 15, 1993 ABSTRACT Moths, butterflies, and grasshoppers were surveyed within four longleaf pine preserves owned by the North Carolina Nature Conservancy during the growing season of 1991 and 1992. Over 7,000 specimens (either collected or seen in the field) were identified, representing 512 different species and 28 families. Forty-one of these we consider to be distinctive of the two fire- maintained communities principally under investigation, the longleaf pine savannas and flatwoods. An additional 14 species we consider distinctive of the pocosins that occur in close association with the savannas and flatwoods. Twenty nine species appear to be rare enough to be included on the list of elements monitored by the North Carolina Natural Heritage Program (eight others in this category have been reported from one of these sites, the Green Swamp, but were not observed in this study). Two of the moths collected, Spartiniphaga carterae and Agrotis buchholzi, are currently candidates for federal listing as Threatened or Endangered species. Another species, Hemipachnobia s. subporphyrea, appears to be endemic to North Carolina and should also be considered for federal candidate status. With few exceptions, even the species that seem to be most closely associated with savannas and flatwoods show few direct defenses against fire, the primary force responsible for maintaining these communities. Instead, the majority of these insects probably survive within this region due to their ability to rapidly re-colonize recently burned areas from small, well-dispersed refugia. -

Orange Sulphur, Colias Eurytheme, on Boneset
Orange Sulphur, Colias eurytheme, on Boneset, Eupatorium perfoliatum, In OMC flitrh Insect Survey of Waukegan Dunes, Summer 2002 Including Butterflies, Dragonflies & Beetles Prepared for the Waukegan Harbor Citizens' Advisory Group Jean B . Schreiber (Susie), Chair Principal Investigator : John A. Wagner, Ph . D . Associate, Department of Zoology - Insects Field Museum of Natural History 1400 South Lake Shore Drive Chicago, Illinois 60605 Telephone (708) 485 7358 home (312) 665 7016 museum Email jwdw440(q-), m indsprinq .co m > home wagner@,fmnh .orq> museum Abstract: From May 10, 2002 through September 13, 2002, eight field trips were made to the Harbor at Waukegan, Illinois to survey the beach - dunes and swales for Odonata [dragonfly], Lepidoptera [butterfly] and Coleoptera [beetles] faunas between Midwest Generation Plant on the North and the Outboard Marine Corporation ditch at the South . Eight species of Dragonflies, fourteen species of Butterflies, and eighteen species of beetles are identified . No threatened or endangered species were found in this survey during twenty-four hours of field observations . The area is undoubtedly home to many more species than those listed in this report. Of note, the endangered Karner Blue butterfly, Lycaeides melissa samuelis Nabakov was not seen even though it has been reported from Illinois Beach State Park, Lake County . The larval food plant, Lupinus perennis, for the blue was not observed at Waukegan. The limestone seeps habitat of the endangered Hines Emerald dragonfly, Somatochlora hineana, is not part of the ecology here . One surprise is the. breeding population of Buckeye butterflies, Junonia coenid (Hubner) which may be feeding on Purple Loosestrife . The specimens collected in this study are deposited in the insect collection at the Field Museum . -

Michigan Butterfly Network Handbook
Michigan Butterfly Network Handbook Kalamazoo Nature Center 2018 Introduction Habitat loss and fragmentation have widespread effects on their respective plant and animal communities. Land managers and stewards must decide how to best approach site restoration, management, and conservation in this new and changing world. Specifically, population decline and species loss are critical components when developing a conservation plan. In Michigan, agricultural land usage has reduced the size of native prairie lands and drained wetlands, both of which are important habitats of plant and animal species, such as native butterflies. Butterflies are charismatic biological indicators that we can use to assess the effects of habitat augmentation and the general health of an ecosystem. Butterflies have unique life histories and specific habitat requirements. Long-term monitoring can be used to assess butterfly status and trends. Abundances can fluctuate from year to year due to sensitivities to climate and habitat structure and a multiple-year approach is necessary to assess how different species of native butterflies are responding to changes occurring in their natural environment over time. We can then use relative population densities of species in the field to assess land management programs and develop butterfly conservation programs. These methods will allow us to uncover population declines before it is too late. How can we monitor and assess butterfly population sizes and trends at a large, statewide scale? We can do this with the help of citizen scientists! We can collect data on butterfly species and populations in a region to gather information on long-term, large-scale trends with our standard monitoring protocol (methodology). -

Species at Risk on Department of Defense Installations
Species at Risk on Department of Defense Installations Revised Report and Documentation Prepared for: Department of Defense U.S. Fish and Wildlife Service Submitted by: January 2004 Species at Risk on Department of Defense Installations: Revised Report and Documentation CONTENTS 1.0 Executive Summary..........................................................................................iii 2.0 Introduction – Project Description................................................................. 1 3.0 Methods ................................................................................................................ 3 3.1 NatureServe Data................................................................................................ 3 3.2 DOD Installations............................................................................................... 5 3.3 Species at Risk .................................................................................................... 6 4.0 Results................................................................................................................... 8 4.1 Nationwide Assessment of Species at Risk on DOD Installations..................... 8 4.2 Assessment of Species at Risk by Military Service.......................................... 13 4.3 Assessment of Species at Risk on Installations ................................................ 15 5.0 Conclusion and Management Recommendations.................................... 22 6.0 Future Directions............................................................................................. -
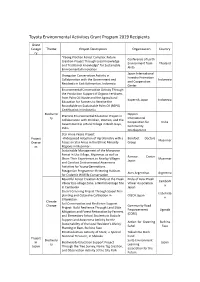
List of Previous Grant Projects
Toyota Environmental Activities Grant Program 2019 Recipients Grant Catego Theme Project Description Organization Country ry "Kaeng Krachan Forest Complex: Future Conference of Earth Creation Project Through Local Knowledge Environment from Thailand and Traditional Knowledge" for Sustainable Akita Environmental Innovation Japan International Orangutan Conservation Activity in Forestry Promotion Collaboration with the Government and Indonesia and Cooperation Residents in East Kalimantan, Indonesia Center Environmental Conservation Activity Through the Production Support of Organic Fertilizers from Palm Oil Waste and the Agricultural Kopernik Japan Indonesia Education for Farmers to Receive the Roundtable on Sustainable Palm Oil (RSPO) Certification in Indonesia Biodiversi Nippon Practical Environmental Education Project in ty International Collaboration with Children, Women, and the Cooperation for India Government in a Rural Village in Bodh Gaya, Community India Development Star Anise Peace Project Project -Widespread Adoption of Agroforestry with a Barefoot Doctors Myanmar Overse Focus on Star Anise in the Ethnic Minority Group as Regions in Myanmar- Sustainable Management of the Mangrove Forest in Uto Village, Myanmar, as well as Ramsar Center Share Their Experiences to Nearby Villages Myanmar Japan and Conduct Environmental Awareness Activities for Young Generations Patagonian Programme: Restoring Habitats Aves Argentinas Argentina for Endemic Wildlife Conservation Beautiful Forest Creation Activity at the Preah Pride of Asia: Preah -

List of Insect Species Which May Be Tallgrass Prairie Specialists
Conservation Biology Research Grants Program Division of Ecological Services © Minnesota Department of Natural Resources List of Insect Species which May Be Tallgrass Prairie Specialists Final Report to the USFWS Cooperating Agencies July 1, 1996 Catherine Reed Entomology Department 219 Hodson Hall University of Minnesota St. Paul MN 55108 phone 612-624-3423 e-mail [email protected] This study was funded in part by a grant from the USFWS and Cooperating Agencies. Table of Contents Summary.................................................................................................. 2 Introduction...............................................................................................2 Methods.....................................................................................................3 Results.....................................................................................................4 Discussion and Evaluation................................................................................................26 Recommendations....................................................................................29 References..............................................................................................33 Summary Approximately 728 insect and allied species and subspecies were considered to be possible prairie specialists based on any of the following criteria: defined as prairie specialists by authorities; required prairie plant species or genera as their adult or larval food; were obligate predators, parasites -

Integrated Noxious Weed Management Plan: US Air Force Academy and Farish Recreation Area, El Paso County, CO
Integrated Noxious Weed Management Plan US Air Force Academy and Farish Recreation Area August 2015 CNHP’s mission is to preserve the natural diversity of life by contributing the essential scientific foundation that leads to lasting conservation of Colorado's biological wealth. Colorado Natural Heritage Program Warner College of Natural Resources Colorado State University 1475 Campus Delivery Fort Collins, CO 80523 (970) 491-7331 Report Prepared for: United States Air Force Academy Department of Natural Resources Recommended Citation: Smith, P., S. S. Panjabi, and J. Handwerk. 2015. Integrated Noxious Weed Management Plan: US Air Force Academy and Farish Recreation Area, El Paso County, CO. Colorado Natural Heritage Program, Colorado State University, Fort Collins, Colorado. Front Cover: Documenting weeds at the US Air Force Academy. Photos courtesy of the Colorado Natural Heritage Program © Integrated Noxious Weed Management Plan US Air Force Academy and Farish Recreation Area El Paso County, CO Pam Smith, Susan Spackman Panjabi, and Jill Handwerk Colorado Natural Heritage Program Warner College of Natural Resources Colorado State University Fort Collins, Colorado 80523 August 2015 EXECUTIVE SUMMARY Various federal, state, and local laws, ordinances, orders, and policies require land managers to control noxious weeds. The purpose of this plan is to provide a guide to manage, in the most efficient and effective manner, the noxious weeds on the US Air Force Academy (Academy) and Farish Recreation Area (Farish) over the next 10 years (through 2025), in accordance with their respective integrated natural resources management plans. This plan pertains to the “natural” portions of the Academy and excludes highly developed areas, such as around buildings, recreation fields, and lawns. -

The Evolutionary Biology of Herbivorous Insects
GRBQ316-3309G-C01[01-19].qxd 7/17/07 12:07 AM Page 1 Aptara (PPG-Quark) PART I EVOLUTION OF POPULATIONS AND SPECIES GRBQ316-3309G-C01[01-19].qxd 7/17/07 12:07 AM Page 2 Aptara (PPG-Quark) GRBQ316-3309G-C01[01-19].qxd 7/17/07 12:07 AM Page 3 Aptara (PPG-Quark) ONE Chemical Mediation of Host-Plant Specialization: The Papilionid Paradigm MAY R. BERENBAUM AND PAUL P. FEENY Understanding the physiological and behavioral mecha- chemistry throughout the life cycle are central to these nisms underlying host-plant specialization in holo- debates. Almost 60 years ago, Dethier (1948) suggested that metabolous species, which undergo complete development “the first barrier to be overcome in the insect-plant relation- with a pupal stage, presents a particular challenge in that ship is a behavioral one. The insect must sense and discrim- the process of host-plant selection is generally carried out inate before nutritional and toxic factors become opera- by the adult stage, whereas host-plant utilization is more tive.” Thus, Dethier argued for the primacy of adult [AQ2] the province of the larval stage (Thompson 1988a, 1988b). preference, or detection and response to kairomonal cues, Thus, within a species, critical chemical, physical, or visual in host-plant shifts. In contrast, Ehrlich and Raven (1964) cues for host-plant identification may differ over the course reasoned that “after the restriction of certain groups of of the life cycle. An organizing principle for the study of insects to a narrow range of food plants, the formerly repel- host-range evolution is the preference-performance hypoth- lent substances of these plants might . -

How to Use This Checklist
How To Use This Checklist Swallowtails: Family Papilionidae Special Note: Spring and Summer Azures have recently The information presented in this checklist reflects our __ Pipevine Swallowtail Battus philenor R; May - Sep. been recognized as separate species. Azure taxonomy has not current understanding of the butterflies found within __ Zebra Swallowtail Eurytides marcellus R; May - Aug. been completely sorted out by the experts. Cleveland Metroparks. (This list includes all species that have __ Black Swallowtail Papilio polyxenes C; May - Sep. __ Appalachian Azure Celastrina neglecta-major h; mid - late been recorded in Cuyahoga County, and a few additional __ Giant Swallowtail Papilio cresphontes h; rare in Cleveland May; not recorded in Cuy. Co. species that may occur here.) Record you observations and area; July - Aug. Brush-footed Butterflies: Family Nymphalidae contact a naturalist if you find something that may be of __ Eastern Tiger Swallowtail Papilio glaucus C; May - Oct.; __ American Snout Libytheana carinenta R; June - Oct. interest. females occur as yellow or dark morphs __ Variegated Fritillary Euptoieta claudia R; June - Oct. __ Spicebush Swallowtail Papilio troilus C; May - Oct. __ Great Spangled Fritillary Speyeria cybele C; May - Oct. Species are listed taxonomically, with a common name, a Whites and Sulphurs: Family Pieridae __ Aphrodite Fritillary Speyeria aphrodite O; June - Sep. scientific name, a note about its relative abundance and flight __ Checkered White Pontia protodice h; rare in Cleveland area; __ Regal Fritillary Speyeria idalia X; no recent Ohio records; period. Check off species that you identify within Cleveland May - Oct. formerly in Cleveland Metroparks Metroparks. __ West Virginia White Pieris virginiensis O; late Apr. -

Whole Genome Shotgun Phylogenomics Resolves the Pattern
Whole genome shotgun phylogenomics resolves the pattern and timing of swallowtail butterfly evolution Rémi Allio, Celine Scornavacca, Benoit Nabholz, Anne-Laure Clamens, Felix Sperling, Fabien Condamine To cite this version: Rémi Allio, Celine Scornavacca, Benoit Nabholz, Anne-Laure Clamens, Felix Sperling, et al.. Whole genome shotgun phylogenomics resolves the pattern and timing of swallowtail butterfly evolution. Systematic Biology, Oxford University Press (OUP), 2020, 69 (1), pp.38-60. 10.1093/sysbio/syz030. hal-02125214 HAL Id: hal-02125214 https://hal.archives-ouvertes.fr/hal-02125214 Submitted on 10 May 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Running head Shotgun phylogenomics and molecular dating Title proposal Downloaded from https://academic.oup.com/sysbio/advance-article-abstract/doi/10.1093/sysbio/syz030/5486398 by guest on 07 May 2019 Whole genome shotgun phylogenomics resolves the pattern and timing of swallowtail butterfly evolution Authors Rémi Allio1*, Céline Scornavacca1,2, Benoit Nabholz1, Anne-Laure Clamens3,4, Felix