Serve You Rx Prior Authorization Information and Drug List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Minutes of the CHMP Meeting 14-17 September 2020
13 January 2021 EMA/CHMP/625456/2020 Corr.1 Human Medicines Division Committee for medicinal products for human use (CHMP) Minutes for the meeting on 14-17 September 2020 Chair: Harald Enzmann – Vice-Chair: Bruno Sepodes Disclaimers Some of the information contained in these minutes is considered commercially confidential or sensitive and therefore not disclosed. With regard to intended therapeutic indications or procedure scopes listed against products, it must be noted that these may not reflect the full wording proposed by applicants and may also vary during the course of the review. Additional details on some of these procedures will be published in the CHMP meeting highlights once the procedures are finalised and start of referrals will also be available. Of note, these minutes are a working document primarily designed for CHMP members and the work the Committee undertakes. Note on access to documents Some documents mentioned in the minutes cannot be released at present following a request for access to documents within the framework of Regulation (EC) No 1049/2001 as they are subject to on- going procedures for which a final decision has not yet been adopted. They will become public when adopted or considered public according to the principles stated in the Agency policy on access to documents (EMA/127362/2006). 1 Addition of the list of participants Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2020. -

2021 National Formulary 3Rd Quarter Edition
2021 National Formulary 3rd Quarter Edition Last Revised: 08/18/2021 Version 2021Q3c Table of Contents OVERVIEW 4 CARDIOVASCULAR (HEART) DRUGS 14 Alpha & Beta Blockers 14 COVERAGE LIMITATION 4 Antihypertensive Combinations 14 Calcium Channel Blockers (CCBs) 14 COMPOUNDED DRUGS 4 ACE Inhibitors without & with Diuretics 15 DRUG PLACEMENT DETERMINATION 4 ACE Inhibitors / CCB Combinations 15 ARBs without & with Diuretics 15 PREFERRED BRAND PRODUCTS 5 ARB Combinations 15 Naprilysin Inhibitors 15 GENERIC SUBSTITUTION 5 Diuretics 15 Renin Inhibtors 16 SINGLE & DUAL SOURCE GENERICS 5 Antiarrhythmics/Anti-Ischemic 16 Cardiac Glycosides 16 PRIOR AUTHORIZATIONS, STEP EDITS & QTY LIMITS 6 Vasodilators, Coronary, Nitrates/Vasodilators, Sympatholytics 16 EXCLUDED DRUGS 6 Other Drugs 16 NON-LISTED DRUGS & DRUG CATEGORIES 7 ANTIHYPERLIPIDEMIC (CHOLESTEROL) DRUGS 17 Statins & Statin/CCB Combinations 17 FORMULARY MODIFICATIONS & CHANGES 7 Bile Acid Sequestrants, Liver Drugs 17 Fibrates 17 BIOSIMILARS 7 ACL Inhibitors 17 Other Drugs 17 MAJOR CHANGES TO THE PDL 7 PANCREATIC DRUGS 18 ANTIBIOTICS 8 Penicillins & Cephalosporins 8 KIDNEY & URINARY / UROLOGICAL DRUGS 18 Tetracyclines 8 Benign Prostate Hyperplasia 18 Macrolides & Clindamycins 8 Urologic Drugs / Other Drugs 18 Sulfonamides, Sulfones & Ketolides 8 Erectile Dysfunction Drugs 18 Quinolones 8 Gout Drugs – Purine Inhibitors 19 Miscellaneous Antibiotics 8 Urinary Ph Modifiers 19 Potassium & Electrolytes 19 ANTI-VIRALS 9 Phosphorus/Calcium/Electrolyte Depleters 19 General Antivirals 9 HIV Antiviral Drugs 9 OSTEOPOROSIS (BONE) DRUGS 20 HIV Pre-Exposure Propylaxis Drugs 9 ANTI-INFLAMMATORY / ANALGESIC (PAIN) DRUGS 20 ANTI-INFECTIVES 10 Anti-Inflammatory Drugs (NSAIDS) 20 Anaerobic Anti-Infectives 10 COX-II Drugs 21 Antiparasitics 10 Analgesics, Narcotics (Opioids) 21 Antimalarials & Antiprotozoals 10 Analgesics, Salicylates, Non-Salicylates, Other 21 Antihelmintic Drugs 10 CENTRAL NERVOUS SYSTEM DRUGS 22 ANTIEMETICS 10 Anti-Anxiety Drugs (Benzodiazepines) 22 Sedative/Sleeping Drugs 22 NEUROLOGIC DRUGS 11 A.D.D. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

European Medicines Agency Accepts Marketing Authorization Application for Asfotase Alfa As a Treatment for Patients with Hypophosphatasia
July 24, 2014 European Medicines Agency Accepts Marketing Authorization Application for Asfotase Alfa as a Treatment for Patients with Hypophosphatasia -- Application designated for review under accelerated assessment process -- CHESHIRE, Conn.--(BUSINESS WIRE)-- Alexion Pharmaceuticals, Inc. (NASDAQ:ALXN) today announced that the Marketing Authorization Application (MAA) for asfotase alfa, an investigational, first-in-class targeted enzyme replacement therapy for the treatment of hypophosphatasia (HPP), has been validated and granted accelerated assessment by the European Medicines Agency (EMA). The acceptance of this MAA marks the beginning of the review process in the European Union (EU) for this potential new treatment. "HPP is a devastating disease for patients and their families due to progressive deterioration of bones and muscle weakness, which can result in impaired respiratory function, severe disability and death," said Leonard Bell, M.D., Chief Executive Officer of Alexion. "If approved, asfotase alfa would be the first therapy for patients with this life-threatening disorder." The EU filing includes positive data from 68 patients with pediatric-onset HPP (ranging from newborns to 66 years of age) enrolled in three pivotal prospective studies and their extensions, as well as a retrospective natural history study in infants. In April, Alexion initiated the rolling submission of a Biologics License Application (BLA) for asfotase alfa as a treatment for patients with HPP with the U.S. Food and Drug Administration (FDA). About -

Keeping up with FDA Drug Approvals: 60 New Drugs in 60 Minutes Elizabeth A
Keeping Up with FDA Drug Approvals: 60 New Drugs in 60 Minutes Elizabeth A. Shlom, PharmD, BCPS Senior Vice President & Director Clinical Pharmacy Program | Acurity, Inc. Privileged and Confidential April 10, 2019 Privileged and Confidential Program Objectives By the end of the presentation, the pharmacist or pharmacy technician participant will be able to: ▪ Identify orphan drugs and first-in-class medications approved by the FDA in 2018. ▪ List five new drugs and their indications. ▪ Identify the place in therapy for three novel monoclonal antibodies. ▪ Discuss at least two new medications that address public health concerns. Dr. Shlom does not have any conflicts of interest in regard to this presentation. Both trade names and generic names will be discussed throughout the presentation Privileged and Confidential 2018 NDA Approvals (NMEs/BLAs) ▪ Lutathera (lutetium Lu 177 dotatate) ▪ Braftovi (encorafenib) ▪ Vizimpro (dacomitinib) ▪ Biktarvy (bictegravir, emtricitabine, ▪ TPOXX (tecovirimat) ▪ Libtayo (cemiplimab-rwic) tenofovir, ▪ Tibsovo (ivosidenib) ▪ Seysara (sarecycline) alafenamide) ▪ Krintafel (tafenoquine) ▪ Nuzyra (omadacycline) ▪ Symdeko (tezacaftor, ivacaftor) ▪ Orilissa (elagolix sodium) ▪ Revcovi (elapegademase-lvir) ▪ Erleada (apalutamide) ▪ Omegaven (fish oil triglycerides) ▪ Tegsedi (inotersen) ▪ Trogarzo (ibalizumab-uiyk) ▪ Mulpleta (lusutrombopag) ▪ Talzenna (talazoparib) ▪ Ilumya (tildrakizumab-asmn) ▪ Poteligeo (mogamulizumab-kpkc) ▪ Xofluza (baloxavir marboxil) ▪ Tavalisse (fostamatinib disodium) ▪ Onpattro (patisiran) -
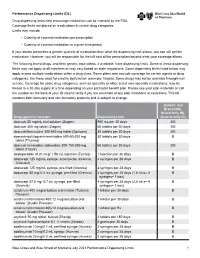
Performance Drug List Dispensing Limits
Performance Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list contains both formulary and non‑formulary products and is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral -

Asfotase Alfa for Infants and Young Children with Hypophosphatasia: 7 Year Outcomes of a Single-Arm, Open-Label, Phase 2 Extension Trial
Articles Asfotase alfa for infants and young children with hypophosphatasia: 7 year outcomes of a single-arm, open-label, phase 2 extension trial Michael P Whyte, Jill H Simmons, Scott Moseley, Kenji P Fujita, Nicholas Bishop, Nada J Salman, John Taylor, Dawn Phillips, Mairead McGinn, William H McAlister Summary Background Our previous phase 2, open-label study of 11 infants and young children with life-threatening perinatal or Lancet Diabetes Endocrinol infantile hypophosphatasia showed 1 year safety and efficacy of asfotase alfa, an enzyme replacement therapy. We 2019; 7: 93–105 aimed to report the long-term outcomes over approximately 7 years of treatment. Published Online December 14, 2018 http://dx.doi.org/10.1016/ Methods We did a prespecified, end of study, 7 year follow-up of our single-arm, open-label, phase 2 trial in which S2213-8587(18)30307-3 children aged 3 years or younger with life-threatening perinatal or infantile hypophosphatasia were recruited from This online publication has been ten hospitals (six in the USA, two in the UK, one in Canada, and one in the United Arab Emirates). Patients received corrected. The corrected version asfotase alfa (1 mg/kg three times per week subcutaneously, adjusted to 3 mg/kg three times per week if required) for first appeared at thelancet. up to 7 years (primary treatment period plus extension phase) or until the product became commercially available; com/diabetes-endocrinology on January 22, 2019 dosage adjustments were made at each visit according to changes in the patient’s weight. The primary objectives of See Comment page 76 this extension study were to assess the long-term tolerability of asfotase alfa, defined as the number of patients with Center for Metabolic Bone one or more treatment-emergent adverse events, and skeletal manifestations associated with hypophosphatasia, Disease and Molecular evaluated using the Radiographic Global Impression of Change (RGI-C) scale (−3 indicating severe worsening, and Research, Shriners Hospital for +3 complete or near-complete healing). -

Asfotase Alfa (Strensiq) for Treatment of Hypophosphatasia in Infants and Children
AHRQ Healthcare Horizon Scanning System – Potential High-Impact Interventions Report Priority Area 08: Functional Limitations and Disability Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 5600 Fishers Lane Rockville, MD 20857 www.ahrq.gov Contract No. HHSA290-2010-00006-C Prepared by: ECRI Institute 5200 Butler Pike Plymouth Meeting, PA 19462 December 2015 Statement of Funding and Purpose This report incorporates data collected during implementation of the Agency for Healthcare Research and Quality (AHRQ) Healthcare Horizon Scanning System by ECRI Institute under contract to AHRQ, Rockville, MD (Contract No. HHSA290-2010-00006-C). The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. This report’s content should not be construed as either endorsements or rejections of specific interventions. As topics are entered into the System, individual topic profiles are developed for technologies and programs that appear to be close to diffusion into practice in the United States. Those reports are sent to various experts with clinical, health systems, health administration, and/or research backgrounds for comment and opinions about potential for impact. The comments and opinions received are then considered and synthesized by ECRI Institute to identify interventions that experts deemed, through the comment process, to have potential for high impact. Please see the methods section for more details about this process. -

Transaction Drug 1St (DIN) 2Nd (PIN) 3Rd (PIN) 4Th (PIN) 5Th (PIN) 6Th
Transaction Drug 1st (DIN) 2nd (PIN) 3rd (PIN) 4th (PIN) 5th (PIN) 6th (PIN) 7th (PIN) 8th (PIN) 9th (PIN) 10th (PIN) 11th (PIN) 12th (PIN) 13th (PIN) Alectinib (Alecensaro®) 02458136 00904400 − − − − − − − − − − − 150 mg capsule Alemtuzumab (LemtradaTM) 02418320 00904161 00904162 00904163 00904164 00904165 00904166 00904167 − − − − − 12 mg / 1.2 mL single-use vial Asfotase alfa (Strensiq®) 02444615 00904483 00904484 00904485 − − − − − − − − − 18 mg / 0.45 mL single-use vial Asfotase alfa (Strensiq®) 02444623 00904486 00904487 00904488 00904489 00904490 − − − − − − − 28 mg / 0.7 mL single-use vial Asfotase alfa (Strensiq®) 02444631 00904491 00904492 00904493 − − − − − − − − − 40 mg / 1 mL single-use vial Asfotase alfa (Strensiq®) 02444658 00904494 00904495 00904496 00904497 00904498 00904499 00904500 00904501 00904502 00904504 00904505 − 80 mg / 0.8 mL single-use vial Canakinumab (Ilaris®) 150 mg/mL powder for solution 02344939 00904404 00903809 00904410 − − − − − − − − − for injection Canakinumab (Ilaris®) 02460351 00904405 00904411 00904412 − − − − − − − − − 150 mg/mL solution for injection Ceftolozane / Tazobactam 02446901 00904433 − − − − − − − − − − − (Zerbaxa®) 1 g / 0.5 g vial Cerliponase Alfa (Brineura®) 150 mg / 5 mL solution for 02484013 00904634 00904635 00904636 − − − − − − − − − intracerebroventricular infusion Cladribine (MavencladTM) 02470179 00904524 00904525 00904526 00904642 − − − − − − − − 10 mg tablet Cysteamine (ProcysbiTM) 02464713 00904354 00904355 − − − − − − − − − − 75 mg delayed-release capsule Daclastavir (DaklinzaTM) -

Gonadotropin Therapy in Assisted Reproduction: an Evolutionary Perspective from Biologics to Biotech
REVIEW Gonadotropin therapy in assisted reproduction: an evolutionary perspective from biologics to biotech Roge´rio de Barros F. Lea˜ o, Sandro C. Esteves Andrology & Human Reproduction Clinic (ANDROFERT), Referral Center for Male Reproduction, Campinas/SP, Brazil. Gonadotropin therapy plays an integral role in ovarian stimulation for infertility treatments. Efforts have been made over the last century to improve gonadotropin preparations. Undoubtedly, current gonadotropins have better quality and safety profiles as well as clinical efficacy than earlier ones. A major achievement has been introducing recombinant technology in the manufacturing processes for follicle-stimulating hormone, luteinizing hormone, and human chorionic gonadotropin. Recombinant gonadotropins are purer than urine- derived gonadotropins, and incorporating vial filling by mass virtually eliminated batch-to-batch variations and enabled accurate dosing. Recombinant and fill-by-mass technologies have been the driving forces for launching of prefilled pen devices for more patient-friendly ovarian stimulation. The most recent developments include the fixed combination of follitropin alfa + lutropin alfa, long-acting FSH gonadotropin, and a new family of prefilled pen injector devices for administration of recombinant gonadotropins. The next step would be the production of orally bioactive molecules with selective follicle-stimulating hormone and luteinizing hormone activity. KEYWORDS: Gonadotropins; Ovulation Induction; Assisted Reproductive Techniques; Systematic Review. -

Infertility Therapy Reference Number: CP.CPA.261 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid – Medi-Cal Revision Log
Clinical Policy: Infertility Therapy Reference Number: CP.CPA.261 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid – Medi-Cal Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description The following are gonadotropins requiring prior authorization: Menotropins (Menopur®), Follitropin alpha, recombinant (Gonal-F® RFF), Follitropin beta, recombinant (Follistim®-AQ), Urofollitropin (Bravelle®), Choriogonadotropin alfa (Ovidrel®), Human chorionic gonadotropin (Novarel®, Pregnyl®), Ganirelex acetate, Cetrorelix (Cetrotide®). FDA approved indication Menopur is indicated for development of multiple follicles and pregnancy in ovulatory women as part of an Assisted Reproductive Technology (ART) cycle. Gonal-F RFF is indicated: • For induction of ovulation and pregnancy in oligo-anovulatory women in whom the cause of infertility is functional and not due to primary ovarian failure. • For development of multiple follicles in ovulatory women as part of an Assisted Reproductive Technology (ART) cycle. Follistim AQ is indicated: • In women for: Induction of ovulation and pregnancy in anovulatory infertile women in whom the cause of infertility is functional and not due to primary ovarian failure. • In women for: Pregnancy in normal ovulatory women undergoing controlled ovarian stimulation as part of an In Vitro Fertilization (IVF) or Intracytoplasmic Sperm Injection (ICSI) cycle. • In men for: Induction of spermatogenesis in men with primary and secondary hypogonadotropic hypogonadism (HH) in whom the cause of infertility is not due to primary testicular failure. Bravelle is indicated: • For induction of ovulation in women who have previously received pituitary suppression. • For development of multiple follicles as part of an Assisted Reproductive Technology (ART) cycle in ovulatory women who have previously received pituitary suppression. -

5.01.610 Pharmacologic Treatment of Infertility
PHARMACY / MEDICAL POLICY – 5.01.610 Pharmacologic Treatment of Infertility Effective Date: Feb. 1, 2021 RELATED MEDICAL POLICIES: Last Revised: Jan. 21, 2021 4.02.503 Infertility and Reproductive Services Replaces: N/A Select a hyperlink below to be directed to that section. POLICY CRITERIA | DOCUMENTATION REQUIREMENTS | CODING RELATED INFORMATION | EVIDENCE REVIEW | REFERENCES | HISTORY ∞ Clicking this icon returns you to the hyperlinks menu above. Introduction Infertility is a problem or problems with the reproductive system that affects the ability to conceive. Different types of reproductive problems affect men and women, but the end result is the inability to conceive or complete a pregnancy. There are many reasons for infertility and drug options vary depending on the cause of infertility and type of infertility treatment required. Even though drug treatment exists, it does not mean it is covered; the member’s contract determines this. This policy describes when infertility drugs may be considered medically necessary if covered by the member’s contract. Note: The Introduction section is for your general knowledge and is not to be taken as policy coverage criteria. The rest of the policy uses specific words and concepts familiar to medical professionals. It is intended for providers. A provider can be a person, such as a doctor, nurse, psychologist, or dentist. A provider also can be a place where medical care is given, like a hospital, clinic, or lab. This policy informs them about when a service may be covered. Policy Coverage