The Auricular Vagus Nerve and Its Influence on Cardiac Control
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sound and the Ear Chapter 2
© Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Chapter© Jones & Bartlett 2 Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Sound and the Ear © Jones Karen &J. Kushla,Bartlett ScD, Learning, CCC-A, FAAA LLC © Jones & Bartlett Learning, LLC Lecturer NOT School FOR of SALE Communication OR DISTRIBUTION Disorders and Deafness NOT FOR SALE OR DISTRIBUTION Kean University © Jones & Bartlett Key Learning, Terms LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR Acceleration DISTRIBUTION Incus NOT FOR SALE OR Saccule DISTRIBUTION Acoustics Inertia Scala media Auditory labyrinth Inner hair cells Scala tympani Basilar membrane Linear scale Scala vestibuli Bel Logarithmic scale Semicircular canals Boyle’s law Malleus Sensorineural hearing loss Broca’s area © Jones & Bartlett Mass Learning, LLC Simple harmonic© Jones motion (SHM) & Bartlett Learning, LLC Brownian motion Membranous labyrinth Sound Cochlea NOT FOR SALE OR Mixed DISTRIBUTION hearing loss Stapedius muscleNOT FOR SALE OR DISTRIBUTION Compression Organ of Corti Stapes Condensation Osseous labyrinth Tectorial membrane Conductive hearing loss Ossicular chain Tensor tympani muscle Decibel (dB) Ossicles Tonotopic organization © Jones Decibel & hearing Bartlett level (dB Learning, HL) LLC Outer ear © Jones Transducer & Bartlett Learning, LLC Decibel sensation level (dB SL) Outer hair cells Traveling wave theory NOT Decibel FOR sound SALE pressure OR level DISTRIBUTION -
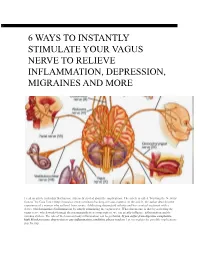
6 Ways to Instantly Stimulate Your Vagus Nerve to Relieve Inflammation, Depression, Migraines and More
O 6 WAYS TO INSTANTLY STIMULATE YOUR VAGUS NERVE TO RELIEVE INFLAMMATION, DEPRESSION, MIGRAINES AND MORE I read an article yesterday that has me extremely excited about the implications. The article is called “Hacking the Nervous System” by Gaia Vince (http://mosaicscience.com/story/hacking-nervous-system). In the article, the author describes the experience of a woman who suffered from severe, debilitating rheumatoid arthritis and her eventual treatment with a device which minimized inflammation by simply stimulating the vagus nerve. What this means, is that by activating the vagus nerve which works through the parasympathetic nervous system, we can greatly influence inflammation and the immune system. The role of the brain on body inflammation can be profound. If you suffer from digestive complaints, high blood pressure, depression or any inflammatory condition, please read on. Let me explain the possible implications step by step. What is the vagus nerve? First of all, the vagus nerve is the longest nerve in the body which originates in the brain as cranial nerve ten, travels down the from go the neck and then passes around the digestive system, liver, spleen, pancreas, heart and lungs. This nerve is a major player in the parasympathetic nervous system, which is the ‘rest and digest’ part (opposite to the sympathetic nervous system which is ‘fight of flight’). Vagal tone The tone of the vagus nerve is key to activating the parasympathetic nervous system. Vagal tone is measured by tracking your heart-rate alongside your breathing rate. Your heart-rate speeds up a little when your breathe in, and slows down a little when you breathe out. -

Questions and Answers About Vagus Nerve Stimulation by Jerry Shih, M.D. 1. WHAT IS VAGUS NERVE STIMULATION? Therapeutic Vagus Ne
Questions and Answers About Vagus Nerve Stimulation By Jerry Shih, M.D. 1. WHAT IS VAGUS NERVE STIMULATION? Therapeutic vagus nerve stimulation (VNS) is chronic, intermittent electrical stimulation of the mid-cervical segment of the left vagus nerve. The stimulation occurs automatically at set intervals, during waking and sleep. The electrical pulses are generated by a pacemaker-like device that is implanted below the clavicle and are delivered by a lead wire that is coiled around the vagus nerve. 2. WHAT IS THE EVIDENCE THAT VAGUS NERVE STIMULATION IS EFFECTIVE IN EPILEPSY? The empirical evidence of antiepileptic efficacy arose sequentially from l) experiments in animal models of epilepsy; 2) anecdotal reports and small case series ofearly human trials, and 3) two prospec-tive, double-blind, controlled studies in large groups of patients with complex partial and secondarily generalized seizures. 3. HOW DOES VAGUS NERVE STIMULATION CONTROL SEIZURES? The mechanisms by which therapeutic VNS reduces seizure activity in humans and in experimental models of epilepsy are unknown. 4. WHEN SHOULD ONE CONSIDER VAGUS NERVE STIMULATION? Medically refractory complex partial and secondarily generalized seizures have been efficaciously treated with adjunctive VNS in the large, randomized studies. Children may benefit considerably from VNS, but large-scale, randomized, controlled studies have not been completed in young children. Thus, any adolescent or adult whose complex partial or secondarily generalized seizures have not been controlled with the appropriate first- and second -line antiepileptic drugs may be a good candidate for VNS. The FDA has specifically approved VNS with the Cyberonics device for adjunctive therapy of refractory partial-onset seizures in persons l2 years of age. -

Instruction Sheet: Otitis Externa
University of North Carolina Wilmington Abrons Student Health Center INSTRUCTION SHEET: OTITIS EXTERNA The Student Health Provider has diagnosed otitis externa, also known as external ear infection, or swimmer's ear. Otitis externa is a bacterial/fungal infection in the ear canal (the ear canal goes from the outside opening of the ear to the eardrum). Water in the ear, from swimming or bathing, makes the ear canal prone to infection. Hot and humid weather also predisposes to infection. Symptoms of otitis externa include: ear pain, fullness or itching in the ear, ear drainage, and temporary loss of hearing. These symptoms are similar to those caused by otitis media (middle ear infection). To differentiate between external ear infection and middle ear infection, the provider looks in the ear with an instrument called an otoscope. It is important to distinguish between the two infections, as they are treated differently: External otitis is treated with drops in the ear canal, while middle ear infection is sometimes treated with an antibiotic by mouth. MEASURES YOU SHOULD TAKE TO HELP TREAT EXTERNAL EAR INFECTION: 1. Use the ear drops regularly, as directed on the prescription. 2. The key to treatment is getting the drops down into the canal and keeping the medicine there. To accomplish this: Lie on your side, with the unaffected ear down. Put three to four drops in the infected ear canal, then gently pull the outer ear back and forth several times, working the medicine deeper into the ear canal. Remain still, good-ear-side-down for about 15 minutes. -

Current Neurosurgical Management of Glossopharyngeal Neuralgia and Technical Nuances for Microvascular Decompression Surgery
Neurosurg Focus 34 (3):E8, 2013 ©AANS, 2013 Current neurosurgical management of glossopharyngeal neuralgia and technical nuances for microvascular decompression surgery ROBERTO REY-DIOS, M.D.,1 AND AARON A. COHEN-GADOL, M.D., M.SC.2 1Department of Neurosurgery, University of Mississippi Medical Center, Jackson, Mississippi; 2Goodman Campbell Brain and Spine, Indiana University Department of Neurological Surgery, Indianapolis, Indiana Glossopharyngeal neuralgia (GPN) is an uncommon facial pain syndrome often misdiagnosed as trigeminal neuralgia. The rarity of this condition and its overlap with other cranial nerve hyperactivity syndromes often leads to a significant delay in diagnosis. The surgical procedures with the highest rates of pain relief for GPN are rhizotomy and microvascular decompression (MVD) of cranial nerves IX and X. Neurovascular conflict at the level of the root exit zone of these cranial nerves is believed to be the cause of this pain syndrome in most cases. Vagus nerve rhizotomy is usually reserved for cases in which vascular conflict is not evident. A review of the literature reveals that although the addition of cranial nerve X rhizotomy may improve the chances of long-term pain control, this maneuver also increases the risk of permanent dysphagia and vocal cord paralysis. The risks of this procedure have to be carefully weighed against its benefits. Based on the authors’ experience, careful patient selection with a thorough exploratory operation most often leads to identification of the site of vascular conflict, obviating the need for cranial nerve X rhizotomy. (http://thejns.org/doi/abs/10.3171/2012.12.FOCUS12391) KEY WORDS • glossopharyngeal neuralgia • microvascular decompression • vagus nerve • rhizotomy • cranial nerve LOSSOPHARYNGEAL neuralgia, or vagoglossopharyn impairment can be found in the distribution of the above geal neuralgia, is a cranial nerve hyperactivity nerves due to structural lesions.20 This classification does pain syndrome leading to severe, transient, sharp not take into consideration associated syncopal events. -
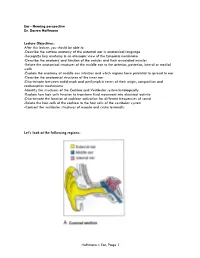
Ear, Page 1 Lecture Outline
Ear - Hearing perspective Dr. Darren Hoffmann Lecture Objectives: After this lecture, you should be able to: -Describe the surface anatomy of the external ear in anatomical language -Recognize key anatomy in an otoscopic view of the tympanic membrane -Describe the anatomy and function of the ossicles and their associated muscles -Relate the anatomical structures of the middle ear to the anterior, posterior, lateral or medial walls -Explain the anatomy of middle ear infection and which regions have potential to spread to ear -Describe the anatomical structures of the inner ear -Discriminate between endolymph and perilymph in terms of their origin, composition and reabsorption mechanisms -Identify the structures of the Cochlea and Vestibular system histologically -Explain how hair cells function to transform fluid movement into electrical activity -Discriminate the location of cochlear activation for different frequencies of sound -Relate the hair cells of the cochlea to the hair cells of the vestibular system -Contrast the vestibular structures of macula and crista terminalis Let’s look at the following regions: Hoffmann – Ear, Page 1 Lecture Outline: C1. External Ear Function: Amplification of Sound waves Parts Auricle Visible part of external ear (pinna) Helix – large outer rim Tragus – tab anterior to external auditory meatus External auditory meatus Auditory Canal/External Auditory Meatus Leads from Auricle to Tympanic membrane Starts cartilaginous, becomes bony as it enters petrous part of temporal bone Earwax (Cerumen) Complex mixture -

Bedside Neuro-Otological Examination and Interpretation of Commonly
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2004.054478 on 24 November 2004. Downloaded from BEDSIDE NEURO-OTOLOGICAL EXAMINATION AND INTERPRETATION iv32 OF COMMONLY USED INVESTIGATIONS RDavies J Neurol Neurosurg Psychiatry 2004;75(Suppl IV):iv32–iv44. doi: 10.1136/jnnp.2004.054478 he assessment of the patient with a neuro-otological problem is not a complex task if approached in a logical manner. It is best addressed by taking a comprehensive history, by a Tphysical examination that is directed towards detecting abnormalities of eye movements and abnormalities of gait, and also towards identifying any associated otological or neurological problems. This examination needs to be mindful of the factors that can compromise the value of the signs elicited, and the range of investigative techniques available. The majority of patients that present with neuro-otological symptoms do not have a space occupying lesion and the over reliance on imaging techniques is likely to miss more common conditions, such as benign paroxysmal positional vertigo (BPPV), or the failure to compensate following an acute unilateral labyrinthine event. The role of the neuro-otologist is to identify the site of the lesion, gather information that may lead to an aetiological diagnosis, and from there, to formulate a management plan. c BACKGROUND Balance is maintained through the integration at the brainstem level of information from the vestibular end organs, and the visual and proprioceptive sensory modalities. This processing takes place in the vestibular nuclei, with modulating influences from higher centres including the cerebellum, the extrapyramidal system, the cerebral cortex, and the contiguous reticular formation (fig 1). -

Hearing Loss, Vertigo and Tinnitus
HEARING LOSS, VERTIGO AND TINNITUS Jonathan Lara, DO April 29, 2012 Hearing Loss Facts S Men are more likely to experience hearing loss than women. S Approximately 17 percent (36 million) of American adults report some degree of hearing loss. S About 2 to 3 out of every 1,000 children in the United States are born deaf or hard-of-hearing. S Nine out of every 10 children who are born deaf are born to parents who can hear. Hearing Loss Facts S The NIDCD estimates that approximately 15 percent (26 million) of Americans between the ages of 20 and 69 have high frequency hearing loss due to exposure to loud sounds or noise at work or in leisure activities. S Only 1 out of 5 people who could benefit from a hearing aid actually wears one. S Three out of 4 children experience ear infection (otitis media) by the time they are 3 years old. Hearing Loss Facts S There is a strong relationship between age and reported hearing loss: 18 percent of American adults 45-64 years old, 30 percent of adults 65-74 years old, and 47 percent of adults 75 years old or older have a hearing impairment. S Roughly 25 million Americans have experienced tinnitus. S Approximately 4,000 new cases of sudden deafness occur each year in the United States. Hearing Loss Facts S Approximately 615,000 individuals have been diagnosed with Ménière's disease in the United States. Another 45,500 are newly diagnosed each year. S One out of every 100,000 individuals per year develops an acoustic neurinoma (vestibular schwannoma). -

Ear Infections
EAR INFECTIONS How common are ear infections in cats? Infections of the external ear canal (outer ear) by bacteria or yeast are common in dogs but not as common in cats. Outer ear infections are called otitis externa. The most common cause of feline otitis externa is ear mite infestation. What are the symptoms of an ear infection? Ear infection cause pain and discomfort and the ear canals are sensitive. Many cats will shake their head and scratch their ears attempting to remove the debris and fluid from the ear canal. The ears often become red and inflamed and develop an offensive odor. A black or yellow discharge is commonly observed. Don't these symptoms usually suggest ear mites? Ear mites can cause several of these symptoms including a black discharge, scratching and head shaking. However, ear mite infections generally occur in kittens. Ear mites in adult cats occur most frequently after a kitten carrying mites is introduced into the household. Sometimes ear mites will create an environment within the ear canal which leads to a secondary infection with bacteria or yeast. By the time the cat is presented to the veterinarian the mites may be gone but a significant ear infection remains. Since these symptoms are similar can I just buy some ear drops? No, careful diagnosis of the exact cause of the problem is necessary to enable selection of appropriate treatment. There are several kinds of bacteria and fungi that might cause an ear infection. Without knowing the kind of infection present, we do not know which drug to use. -

Specialized for Sound Detection A. Outer
EAR © 2019zillmusom I. Overview - specialized for sound detection A. Outer ear - funnel shaped structure of cartilage and skin that leads to Tympanic membrane; directs sound toward Tympanic membrane; helps detect source of sound. B. Middle ear - air filled chamber that contains bones (ossicles) that link Tympanic membrane to cochlea; also contains muscles that dampen sounds; middle ear is linked to Nasopharynx by auditory tube which allows for equilibration of air pressure on inner side of Tympanic membrane. C. Inner ear - fluid filled chamber in petrous part of temporal bone; inner ear contains Cochlea (hearing) and Vestibular apparatus for gravity detection (both innervated by CN VIII). Clinical Note: Functioning of inner ear can be tested independently by vibrations transmitted directly through bone (Weber test: tuning fork on calvarium is perceived as sound); CONDUCTIVE HEARING LOSS - damage to middle ear (tympanic membrane, auditory ossicles); SENSORINEURAL HEARING LOSS - damage to inner ear (cochlea, CN VIII). II. Outer Ear - composed of two parts: A. Auricle (pinna) - elastic cartilage covered with skin; functions to reflect sound waves. Parts: helix, antihelix, tragus and lobule. Decorative Note: Cartilage does not extend into Lobule; Lobule can be readily pierced to provide support for decorative metal objects. B. External auditory meatus - tube from auricle to the Tympanic membrane; posterior to Parotid gland and TMJ (Temporomandibular joint); located anterior to mastoid process. Outer third consists of elastic cartilage; contains hairs, sebaceous glands and ceruminous glands (produce cerumen = ear wax); serves to protect Tympanic membrane; Inner two thirds is composed of bone lined with skin. Clinical note: External auditory meatus is curved anteriorly in adults, is straight in children; in adults, auricle is pulled up and back to insert otoscope. -

ANATOMY of EAR Basic Ear Anatomy
ANATOMY OF EAR Basic Ear Anatomy • Expected outcomes • To understand the hearing mechanism • To be able to identify the structures of the ear Development of Ear 1. Pinna develops from 1st & 2nd Branchial arch (Hillocks of His). Starts at 6 Weeks & is complete by 20 weeks. 2. E.A.M. develops from dorsal end of 1st branchial arch starting at 6-8 weeks and is complete by 28 weeks. 3. Middle Ear development —Malleus & Incus develop between 6-8 weeks from 1st & 2nd branchial arch. Branchial arches & Development of Ear Dev. contd---- • T.M at 28 weeks from all 3 germinal layers . • Foot plate of stapes develops from otic capsule b/w 6- 8 weeks. • Inner ear develops from otic capsule starting at 5 weeks & is complete by 25 weeks. • Development of external/middle/inner ear is independent of each other. Development of ear External Ear • It consists of - Pinna and External auditory meatus. Pinna • It is made up of fibro elastic cartilage covered by skin and connected to the surrounding parts by ligaments and muscles. • Various landmarks on the pinna are helix, antihelix, lobule, tragus, concha, scaphoid fossa and triangular fossa • Pinna has two surfaces i.e. medial or cranial surface and a lateral surface . • Cymba concha lies between crus helix and crus antihelix. It is an important landmark for mastoid antrum. Anatomy of external ear • Landmarks of pinna Anatomy of external ear • Bat-Ear is the most common congenital anomaly of pinna in which antihelix has not developed and excessive conchal cartilage is present. • Corrections of Pinna defects are done at 6 years of age. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria.