Nordic Nutrition Recommendations 2012 Integrating Nutrition and Physical Activity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Knowledge-To-Action Approach to Food Fortification: Guiding Principles for the Design of Fortification Programs As a Means Of
Vol.4, No.10, 904-909 (2012) Health http://dx.doi.org/10.4236/health.2012.410138 A knowledge-to-action approach to food fortification: Guiding principles for the design of fortification programs as a means of effectively addressing micronutrient malnutrition Laura A. Rowe*, David M. Dodson Project Healthy Children, Cambridge, USA; *Corresponding Author: [email protected] Received 28 August 2012; revised 26 September 2012; accepted 5 October 2012 ABSTRACT clear and concise messages or frameworks that allow for scaling-up in changing, challenging, and dynamic envi- The gap that exists between research and the ronments [8]. dissemination and implementation of research This paper seeks to add to the already existing litera- findings has been well established. Food forti- ture on how to implement national food fortification pro- fication, one of the most cost-effective means of grams in a way that addresses the “knowing-doing” gap addressing micronutrient malnutrition, is no ex- by offering a streamlined model that facilitates moving ception. With decades of implementation experi- from evidence to application. Based on Project Healthy ence, there is need to strengthen mechanisms Children’s (PHC) experience assisting governments in that effectively broadcast proven strategies to the design and implementation of national food fortifica- promote the successful implementation of forti- tion programs in Rwanda and Malawi, in this paper we 1) fication programs in changing, challenging, and describe how nationwide, mandatory food fortification dynamic environments. This requires clear chan- programs, often missing from national agendas, are dis- nels of communication, well-defined in-country tinct in how they address the problem of malnutrition; leadership, and a streamlined and focused ap- and 2) document four core, arguably intangible and often proach that can be adapted to country-specific overlooked, principles needed to solidify engagement, contexts. -

Petrography, Geochemistry and Genesis of the Skiftesmyr Cu-Zn VMS- Deposit, Grong, Norway
FACULTY OF SCIENCE AND TECHNOLOGY DEPARTMENT OF GEOLOGY Petrography, geochemistry and genesis of the Skiftesmyr Cu-Zn VMS- deposit, Grong, Norway Kristoffer Jøtne Walsh GEO-3900 Master’s Thesis in Geology November 2013 GEO-3900 Master’s Thesis in Geology PETROGRAPHY, GEOCHEMISTRY AND GENESIS OF THE SKIFTESMYR CU-ZN VMS-DEPOSIT, GRONG, NORWAY Kristoffer Jøtne Walsh Department of Geology, UiT – The Arctic University of Norway, November, 2013 2 3 Acknowledgements I wish to thank my supervisors, Professor Kåre V. Kullerud and Perry O. Kaspersen, former CEO and Country Manager of MetPro AS, for all their help and comments, and MetPro AS for making this thesis possible. I would also like to thank Professor Erling Krogh Ravna for assistance with the XRF analyses, and express my gratitude to former MetPro AS geologist Stefan Winterhoff for assistance with field work, and Per Samskog, MetPro AS exploration geologist, for MapInfo tutoring. Last, but not least, I would like to thank my family, friends and fellow students for their support and encouragement. Kristoffer Jøtne Walsh Tromsø, Nov. 2013 4 5 Abstract The Skiftesmyr Cu-Zn VMS-deposit is located in the Grong municipality of Northern Trøndelag, Norway. The mineralization has been known since at least 1903, when mention of small workings in the area were first published, and has later been the subject of several exploration projects by different companies, of which MetPro AS is the latest. The Skiftesmyr deposit is a part of the Gjersvik Nappe, which is a part of the Köli Nappe Complex, which in turn is a part of the Upper Allochthon of the Scandinavian Caledonides, and is likely of Mid- Ordovician age. -

Fortified Food and Supplements: Approaches for Long-Term Care Residents
ASK AN EXPERT DAVID PAPAZIAN/SHUTTERSTOCK.COM Fortified Food and Supplements: Approaches for Long-Term Care Residents By Julie Cavaliere, “To cure sometimes, to relieve often, to comfort always”. Ba.Sc., RD, Manager of –Hippocrates Dietary, Nutrition and ood and dining are key components of quality of life and Environmental Operations quality of care for residents in long-term care homes. for Sienna Senior Living The prevalence of protein energy under-nutrition for residents ranges from 23 per cent to 85 per cent, making malnutrition one of the most serious problems facing health professionals in long-term care. Malnutrition is associated with poor outcomes and is an indicator of risk for increased mortality. It has been found that most residents with evi- dence of malnutrition were on restricted diets that might discourage nutrient intake.1 Researchers, Nutrition Managers, and Registered Dietitians alike have been looking at ways and methods to fortify foods without increasing the volume of food consumed. What exactly is a fortified food? Health Canada defines food fortification as “a process by which vitamins, mineral nutrients and amino acids are added to foods to provide consumers with sufficient but not excessive amounts of certain nutrients in their diet.”2 There is both mandatory and voluntary fortification of foods at present. Mandatory fortification includes addition of vitamin A and D to milk, CANADIAN SOCIETY OF NUTRITION MANAGEMENT NEWS – WINTER 2018 11 for example. Meal replacements or nutritional supplements of a known food, cost, acceptance, not a normalized way of for the purposes of this article can be defined “a formulated eating, and no connection to the past. -

Guidelines on Food Fortification with Micronutrients
GUIDELINES ON FOOD FORTIFICATION FORTIFICATION FOOD ON GUIDELINES Interest in micronutrient malnutrition has increased greatly over the last few MICRONUTRIENTS WITH years. One of the main reasons is the realization that micronutrient malnutrition contributes substantially to the global burden of disease. Furthermore, although micronutrient malnutrition is more frequent and severe in the developing world and among disadvantaged populations, it also represents a public health problem in some industrialized countries. Measures to correct micronutrient deficiencies aim at ensuring consumption of a balanced diet that is adequate in every nutrient. Unfortunately, this is far from being achieved everywhere since it requires universal access to adequate food and appropriate dietary habits. Food fortification has the dual advantage of being able to deliver nutrients to large segments of the population without requiring radical changes in food consumption patterns. Drawing on several recent high quality publications and programme experience on the subject, information on food fortification has been critically analysed and then translated into scientifically sound guidelines for application in the field. The main purpose of these guidelines is to assist countries in the design and implementation of appropriate food fortification programmes. They are intended to be a resource for governments and agencies that are currently implementing or considering food fortification, and a source of information for scientists, technologists and the food industry. The guidelines are written from a nutrition and public health perspective, to provide practical guidance on how food fortification should be implemented, monitored and evaluated. They are primarily intended for nutrition-related public health programme managers, but should also be useful to all those working to control micronutrient malnutrition, including the food industry. -

South African Food-Based Dietary Guidelines
ISSN 1607-0658 Food-Based Dietary Guidelines for South Africa S Afr J Clin Nutr 2013;26(3)(Supplement):S1-S164 FBDG-SA 2013 www.sajcn.co.za Developed and sponsored by: Distribution of launch issue sponsored by ISSN 1607-0658 Food-Based Dietary Guidelines for South Africa S Afr J Clin Nutr 2013;26(3)(Supplement):S1-S164 FBDG-SA 2013 www.sajcn.co.za Table of contents Cited as: Vorster HH, Badham JB, Venter CS. An 9. “Drink lots of clean, safe water”: a food-based introduction to the revised food-based dietary guidelines dietary guideline for South Africa Van Graan AE, Bopape M, Phooko D, for South Africa. S Afr J Clin Nutr 2013;26(3):S1-S164 Bourne L, Wright HH ................................................................. S77 Guest Editorial 10. The importance of the quality or type of fat in the diet: a food-based dietary guideline for South Africa • Revised food-based dietary guidelines for South Africa: Smuts CM, Wolmarans P.......................................................... S87 challenges pertaining to their testing, implementation and evaluation 11. Sugar and health: a food-based dietary guideline Vorster HH .................................................................................... S3 for South Africa Temple NJ, Steyn NP .............................................................. S100 Food-Based Dietary Guidelines for South Africa 12. “Use salt and foods high in salt sparingly”: a food-based dietary guideline for South Africa 1. An introduction to the revised food-based dietary Wentzel-Viljoen E, Steyn K, Ketterer E, Charlton KE ............ S105 guidelines for South Africa Vorster HH, Badham JB, Venter CS ........................................... S5 13. “If you drink alcohol, drink sensibly.” Is this 2. “Enjoy a variety of foods”: a food-based dietary guideline still appropriate? guideline for South Africa Jacobs L, Steyn NP................................................................ -

294 002 Myrene I Fillan Herred Sør-Trøndelag Fylke
MYRENE, I FILLAN HERRED 85 MYRENE I FILLAN HERRED, SØR-TRØNDELAG FYLKE. Av konsulent Ose. Hovde. Fl 11 an herred består av nordøstre del av øya Hitra og dessuten av flere mindre øyer og holmer nord-Øst for Hitra i sør-Trøndelag fylke. Geografisk sett ligger herredet mellom 63° 32' 42" og 63° 44' 41" nordlige bredde og 1 ° 26' 24" og 1 ° 50' 9'' vestlig lengde, regnet fra Oslo meridian. Fillan grenser til herredene Sandstad i sør og Hitra i vest. I nord og øst begrenses herredet av hav, nemlig henholdsvis Frøysjøen og Frohavet. De nærmeste herreder er: I nord Nord-Frøya og i øst Ørland. Fillfjorden danner skille mellom Hitra og herredets andre Øyer, hvorav Fjellværøy og Ulvøy er de største. Herredets totalareal er 113,38 km- og landarealet utgjør 108,72 km2• Herav ligger omtrent ¾ på Hitra. Fillan har småkupert landskap, men ingen hØye fjell. De største høyder går opp til ca. 180 mo. h. Langs grensen mot Sandstad finnes en del furuskog, men ellers er de mindre øyer, og største delen av her• redet for øvrig, så godt som skogbart. Jordbruks te 11 ingen av 1949 oppgir ca. 1 km2 skog (ve• sentlig barskog) på de bruk som er med i tellingen, mens skogtellingen regner med ca. 6,5 km2 skog i hele herredet. Av udyrket, dyrkbar fast• marksjord oppgir foran nevnte jordbrukstelling 468 dekar, og av myr 1317 dekar. Jordbruksarealet er oppført med vel 3800 dekar, hvorav vel 3000 dekar er dyrket. Dette areal er fordelt på 188 bruk. Gjennom• snittsstørrelsen på brukene blir således ca. -

The Unfinished Agenda for Food Fortification in Low-And Middle
nutrients Review The Unfinished Agenda for Food Fortification in Low- and Middle-Income Countries: Quantifying Progress, Gaps and Potential Opportunities Penjani Mkambula 1,*, Mduduzi N. N. Mbuya 1,* , Laura A. Rowe 2 , Mawuli Sablah 3, Valerie M. Friesen 1 , Manpreet Chadha 4, Akoto K. Osei 5, Corinne Ringholz 6, Florencia C. Vasta 1 and Jonathan Gorstein 7 1 Global Alliance for Improved Nutrition, Rue de Varembé 7, 1202 Geneva, Switzerland; [email protected] (V.M.F.); [email protected] (F.C.V.) 2 Food Fortification Initiative, 1518 Clifton Road, Atlanta, GA 30322, USA; laura.rowe@ffinetwork.org 3 UNICEF, 3 UN Plaza, New York, NY 10017, USA; [email protected] 4 Nutrition International 180 Elgin St., Suite 1000, Ottawa, ON K2P 2K3, Canada; [email protected] 5 Helen Keller International, Regional Office for Africa, Dakar BP 29.898, Senegal; [email protected] 6 World Food Programme, Via Cesare Giulio Viola, 68, 00148 Rome, Italy; [email protected] 7 Iodine Global Network, Seattle, WA 98107, USA; [email protected] * Correspondence: [email protected] (P.M.); [email protected] Received: 31 December 2019; Accepted: 27 January 2020; Published: 29 January 2020 Abstract: Large-scale food fortification (LSFF) is a cost-effective intervention that is widely implemented, but there is scope to further increase its potential. Toidentify gaps and opportunities, we first accessed the Global Fortification Data Exchange (GFDx) to identify countries that could benefit from new fortification programs. Second, we aggregated Fortification Assessment Coverage Toolkit (FACT) survey data from 16 countries to ascertain LSFF coverage and gaps therein. Third, we extended our narrative review to assess current innovations. -

Planbeskrivelse-1.Pdf
Oppdragsgiver Hitra Kommune Rapporttype Planbeskrivelse 15.09.2015 PLANBESKRIVELSE TIL DETALJREGULERING AV JØSTENØY INDUSTRIOMRÅDE JØSTENØY INDUSTRIOMRÅDE 3 (35) PLANBESKRIVELSE TIL DETALJREGULERING AV JØSTENØY INDUSTRIOMRÅDE Oppdragsnr.: 135 000 7205 Oppdragsnavn: Dokument nr.: 1 Filnavn: Planbeskrivelse - Jøstenøy Revisjon 000 001 002 003 Dato 2015-09-15 2015-12-07 Utarbeidet av Lars Arne Bø, Lars Arne Bø Dorte Solvang Kontrollert av Eirik Lind Godkjent av Eirik Lind Revisjonsoversikt Revisjon Dato Revisjonen gjelder 1 07.12.2015 Endringer etter innspill fra Hitra kommune Rambøll Mellomila 79 4 (35) JØSTENØY INDUSTRIOMRÅDE INNHOLD 1. BAKGRUNN .............................................................................. 6 1.1 Hensikten med planen ................................................................ 6 1.2 Forslagsstiller/eierforhold, plankonsulent ...................................... 6 2. PLANPROSESSEN .................................................................... 6 2.1 Varsel om oppstart og planprosess ............................................... 6 2.2 Krav om konsekvensutredning ..................................................... 7 2.3 Innspill ..................................................................................... 7 3. PLANSTATUS OG RAMMEBETINGELSER ................................... 8 3.1 Statlige planer og føringer ........................................................... 8 3.2 Kommunale (overordnede) planer ................................................ 8 3.3 Gjeldnede reguelringsplaner -

Årbok for Fosen – Årbokartikler Alfabetisk
Artikler i Årbok for Fosen – alfabetisk fra Årsskrift 1947 til Årbok 2020 Ved Eilert Bjørkvik og Johan G Foss Bruk CTRL F for å søke etter ord i lista Forfatter Tittel År og side Aksdal, Bjørn En samling folketoner fra Bjørnør, nedskrevet av Mathias A. Lothe 1985:61 Oløkko fer både her og der. Et dikt fra omgangsskolens tid i Alstad, Karin 1996:77 Snillfjord Alsvik, Elling Om bygdemuseer 1971:7 Interiør og inventar på Austrått. En vandring gjennom slottet på Andersen, Eystein M 2004:7 1700-tallet Andersen, Håkon og Hemmelige katolikker. Et kryptokatolsk miljø omkring kanslerne 2001:123 Bratberg, Terje T. V. Bjelke på 1600-tallet Andersen, Steinar Lekterbygging i Brattåa 2000:105 Andresen, Harald Tragedien utenfor Harbak i 1978 2016:63 Andresen, Harald Staværingen – båten som ble en legende 2017:63 Andresen, Harald Dueskar-grenda i Stjørna før i tia 2018:7 Andresen, Harald Historien om Olbert Fredriksen 2020:077 Andresen, Øystein Staværingen og stormen. Et reiseminne fra 1939 2006:121 Aune, Arnfinn Sauehald og slakting på Hitra 1974:191 Aune, Arnfinn Frå børtre til vassverk på Sandstad 1975:109 Aune, Arnfinn Tresko på Hitra 1976:115 Aune, Arnfinn Hitterværingane og feitsilda 1977:17 Aune, Arnfinn Fosentonane. Natur, folkeliv og ferdsel 1990:7 Aune, Arnfinn Storlygaren. Ein mann av reisande slekt 1991:39 Aune, Arnfinn Hitterværingar i ver og vind 1992:53 Aune, Arnfinn Ordtak frå Hitra 1993:151 Aune, Arnfinn Biletspråk og kallsmål på Trøndelagskysten 1996:113 Aune, Arnfinn Biletspråk frå husdyrhaldet 1997:25 Aune, Arnfinn Ordbilete frå husdyrmiljø 2000:79 Aune, Egil Attåtfor i Fosen 1967:68 Aune, Egil ”Ta i og dra!” – Fra Sørfjord meieri før motoren 1991:43 Aune, Egil Minner fra Holvatnet 2002:65 Aune, Hallgerd og Kjerkgangand kle. -
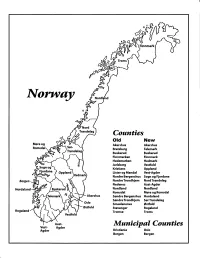
Norway Maps.Pdf
Finnmark lVorwny Trondelag Counties old New Akershus Akershus Bratsberg Telemark Buskerud Buskerud Finnmarken Finnmark Hedemarken Hedmark Jarlsberg Vestfold Kristians Oppland Oppland Lister og Mandal Vest-Agder Nordre Bergenshus Sogn og Fjordane NordreTrondhjem NordTrondelag Nedenes Aust-Agder Nordland Nordland Romsdal Mgre og Romsdal Akershus Sgndre Bergenshus Hordaland SsndreTrondhjem SorTrondelag Oslo Smaalenenes Ostfold Ostfold Stavanger Rogaland Rogaland Tromso Troms Vestfold Aust- Municipal Counties Vest- Agder Agder Kristiania Oslo Bergen Bergen A Feiring ((r Hurdal /\Langset /, \ Alc,ersltus Eidsvoll og Oslo Bjorke \ \\ r- -// Nannestad Heni ,Gi'erdrum Lilliestrom {", {udenes\ ,/\ Aurpkog )Y' ,\ I :' 'lv- '/t:ri \r*r/ t *) I ,I odfltisard l,t Enebakk Nordbv { Frog ) L-[--h il 6- As xrarctaa bak I { ':-\ I Vestby Hvitsten 'ca{a", 'l 4 ,- Holen :\saner Aust-Agder Valle 6rrl-1\ r--- Hylestad l- Austad 7/ Sandes - ,t'r ,'-' aa Gjovdal -.\. '\.-- ! Tovdal ,V-u-/ Vegarshei I *r""i'9^ _t Amli Risor -Ytre ,/ Ssndel Holt vtdestran \ -'ar^/Froland lveland ffi Bergen E- o;l'.t r 'aa*rrra- I t T ]***,,.\ I BYFJORDEN srl ffitt\ --- I 9r Mulen €'r A I t \ t Krohnengen Nordnest Fjellet \ XfC KORSKIRKEN t Nostet "r. I igvono i Leitet I Dokken DOMKIRKEN Dar;sird\ W \ - cyu8npris Lappen LAKSEVAG 'I Uran ,t' \ r-r -,4egry,*T-* \ ilJ]' *.,, Legdene ,rrf\t llruoAs \ o Kirstianborg ,'t? FYLLINGSDALEN {lil};h;h';ltft t)\l/ I t ,a o ff ui Mannasverkl , I t I t /_l-, Fjosanger I ,r-tJ 1r,7" N.fl.nd I r\a ,, , i, I, ,- Buslr,rrud I I N-(f i t\torbo \) l,/ Nes l-t' I J Viker -- l^ -- ---{a - tc')rt"- i Vtre Adal -o-r Uvdal ) Hgnefoss Y':TTS Tryistr-and Sigdal Veggli oJ Rollag ,y Lvnqdal J .--l/Tranbv *\, Frogn6r.tr Flesberg ; \. -

New Techniques and Methods for Decreasing Healthy Tissue Dose in Prostate Cancer Radiotherapy, with Special Reference to Rectal Doses
D 1364 OULU 2016 D 1364 UNIVERSITY OF OULU P.O. Box 8000 FI-90014 UNIVERSITY OF OULU FINLAND ACTA UNIVERSITATIS OULUENSIS ACTA UNIVERSITATIS OULUENSIS ACTA DMEDICA Vesa-Pekka Heikkilä Vesa-Pekka Heikkilä Vesa-Pekka Professor Esa Hohtola NEW TECHNIQUES AND University Lecturer Santeri Palviainen METHODS FOR DECREASING Postdoctoral research fellow Sanna Taskila HEALTHY TISSUE DOSE IN PROSTATE CANCER Professor Olli Vuolteenaho RADIOTHERAPY, WITH University Lecturer Veli-Matti Ulvinen SPECIAL REFERENCE TO RECTAL DOSES Director Sinikka Eskelinen Professor Jari Juga University Lecturer Anu Soikkeli Professor Olli Vuolteenaho UNIVERSITY OF OULU GRADUATE SCHOOL; UNIVERSITY OF OULU, FACULTY OF MEDICINE; Publications Editor Kirsti Nurkkala MEDICAL RESEARCH CENTER OULU; OULU UNIVERSITY HOSPITAL ISBN 978-952-62-1207-4 (Paperback) ISBN 978-952-62-1208-1 (PDF) ISSN 0355-3221 (Print) ISSN 1796-2234 (Online) ACTA UNIVERSITATIS OULUENSIS D Medica 1364 VESA-PEKKA HEIKKILÄ NEW TECHNIQUES AND METHODS FOR DECREASING HEALTHY TISSUE DOSE IN PROSTATE CANCER RADIOTHERAPY, WITH SPECIAL REFERENCE TO RECTAL DOSES Academic dissertation to be presented with the assent of the Doctoral Training Committee of Health and Biosciences of the University of Oulu for public defence in Auditorium 7 of Oulu University Hospital, on 10 June 2016, at 12 noon UNIVERSITY OF OULU, OULU 2016 Copyright © 2016 Acta Univ. Oul. D 1364, 2016 Supervised by Docent Antero Koivula Docent Markku Vaarala Docent Juha Nikkinen Reviewed by Docent Tapani Lahtinen Professor Jarmo Kulmala Opponent Docent Simo Hyödynmaa ISBN 978-952-62-1207-4 (Paperback) ISBN 978-952-62-1208-1 (PDF) ISSN 0355-3221 (Printed) ISSN 1796-2234 (Online) Cover Design Raimo Ahonen JUVENES PRINT TAMPERE 2016 Heikkilä, Vesa-Pekka, New techniques and methods for decreasing healthy tissue dose in prostate cancer radiotherapy, with special reference to rectal doses. -

Review of Cereal Growing in Shetland
REVIEW OF CEREAL GROWING IN SHETLAND Bere Growing At Bigton Farm In Shetland By Peter Martin Agronomy Institute Orkney College (University of the Highlands and Islands) April 2015 Review Of Cereal Growing In Shetland _________________________________________________________________________ Contents Executive Summary ...................................................................................................................... 3 1 Introduction ......................................................................................................................... 8 2 Cereal Growing In Shetland ................................................................................................... 9 2.1 Location ........................................................................................................................ 9 2.2 Recent Weather ............................................................................................................ 9 2.2.1 Temperature .......................................................................................................... 9 2.2.2 Growing Degree Days ........................................................................................... 10 2.2.3 Rainfall ................................................................................................................ 12 2.2.4 Sunshine .............................................................................................................. 13 2.3 Geology And Soil ........................................................................................................