Earth's Place in the Universe
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
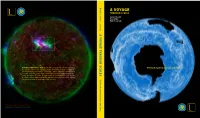
A Voyage Through Scales Zoom Into a Cloud
vv Blöschl A VOYAGE T THROUGH SCALES est. 2002 hybo Günter Blöschl Hans Thybo S Hubert Savenije avenije A VOY A GE THROUGH SC GE THROUGH A VOYAGE THROUGH SCALES Zoom into a cloud. Zoom out of a rock. Watch The Earth System in Space and Time the volcano explode, the lightning strike, an aurora undulate. Imagine ice A sheets expanding, retreating – pulsating – while continents continue their LES leisurely collisions. Everywhere there are structures within structures … within structures. A Voyage Through Scales is an invitation to contemplate the Earth’s extraordinary variability extending from milliseconds to billions of years, from microns to the size of the universe. T he E arth S ystem in ystem S pace and pace T ime est. 2002 T 2 A Voyage Through Scales A Voyage Through Scales 3L A VOYAGE THROUGH SCALES The Earth System in Space and Time T 4 A Voyage Through Scales A Voyage Through Scales L1 PREFACE Patterns of billions of stars on the night skies, cloud patterns, sea ice whirling in the ocean, rivers meandering in the landscape, vegetation patterns on hillslopes, minerals glittering in the sun, and the remains of miniature crea- tures in rocks – they all reveal themselves as complex patterns from the scale of the universe down to the molecu- lar level. A voyage through space scales. From a molten Earth to a solid crust, the evolution and extinction of species, climate fluctuations, continents moving around, the growth and decay of ice sheets, the water cycle wearing down mountain ranges, volcanoes exploding, forest fires, avalanches, sudden chemical reactions – constant change taking place over billions of years down to milliseconds. -

Clinical Update
Clinical Update Femtosecond Laser Brings New Level of Precision to Cataract Procedures The femtosecond laser has increased the the time the eye is open and eases stress on accuracy of the most common surgical the eye’s internal structures. And with such procedure in the United States, according to accuracy at our disposal, we anticipate the laser the surgeon who was instrumental in bringing will open new avenues of treatment that have the advanced tool to the UCLA Stein Eye never been possible before.” Institute last year. Surgeons at Stein Eye have been using the The Alcon LenSx, now used for precision cataract femtosecond laser to assist with several procedures at the Institute’s outpatient surgical steps of cataract surgery, including corneal center, emits optical pulses at the unimaginably incisions to remove the cataract and manage short duration of a femtosecond––one-millionth astigmatism, lens softening, and making an of one-billionth of a second. opening in the capsular bag. “A femtosecond laser can be thought of as For cataract procedures, the femtosecond laser a microscalpel, incising the cornea and lens system is gently docked to the patient’s eye capsule and breaking up the cataract on a and optical coherence tomography imaging Dr. Kevin Miller uses the Alcon LenSx femtosecond laser microscopic scale with an incredible level is used to map the eye’s internal structures. to assist with several steps of cataract surgery, under of precision,” says Kevin M. Miller, MD, Before the operation, the surgeon programs imaging guidance. The femtosecond laser enables Kolokotrones Chair in Ophthalmology. “With the location and size of the incisions as well physicians in the UCLA Stein Eye Institute’s new outpatient surgical center to operate more efficiently a femtosecond laser, I can operate more as the region of the lens to be softened. -

An Ultrasound Portrait of the Embryonic Universe by an Dre W E, Lange
\'1t han~ a cle" \,ICW. ha c k to that moment turn", , ransparcn!. By analog In cosmic h"'StofY when lh·e Ulll,-. .. rSIe y !O a human lifetime .. afler concepdon_b. rh,s IS about six hours r t,orc tht· l Yt;ore• has di"idt-d r:or t IIt fnst. time. ,. H~I~i!lI~G & \ { I E Nt E ... J !OOO An Ultrasound Portrait of the Embryonic Universe by An dre w E, Lange I've been working wirh relescopes carried aloft In fact, the woodcut is preny accurate-there by balloons since I was a graduate studem, We'd is a beyond, and in order to lead yo u to it, [ 'II have launch chern from Palestine, Texas , and my job to take you on a brief and rarher Caltech·cemric was to dri ve like a bat Oll[ of hell all night long tOur of modern cosmology. (That's nor hard to do, to Tuscaloosa, Alabama, to sec up rhe downrange beclluse Gltech has played a remarkably impor srarion, Then, when che balloon lost mdio coman ram role,) ['II talk llbom three seminal observa with Texas, I could receive rhe data in Alabama, tions. The fiTS[ was made by Edwin H ubble L ~ft: What would w~ s~e if And, as will nor surprise my wife, I was frequeml y at the t.k Wilson Observatory, which overlooks we (ould look beyond the scoPlx·d for spel1:ling--once in Louisiana, which is Pasadena, in 1929, Seveml people, narably Vesco visible heavens? something you never, ever want to have happen to Melvin Slipher, had noticed that the galaxies out you. -

Stochastic Model for the Chey-P Molarity in the Neighbourhood of E
Stochastic model for the CheY-P molarity in the neighbourhood of E. coli flagella motors G. Fier1, D. Hansmann∗1,2, and R. C. Buceta†1,2 1Instituto de Investigaciones F´ısicasde Mar del Plata, UNMdP and CONICET 2Departamento de F´ısica,FCEyN, Universidad Nacional de Mar del Plata Funes 3350, B7602AYL Mar del Plata, Argentina November 5, 2019 Abstract Escherichia coli serves as prototype for the study of peritrichous enteric bacteria that perform runs and tumbles alternately. Bacteria run forward as a result of the counterclockwise (CCW) rotation of their flagella bundle, which is located rearward, and perform tumbles when at least one of their flagella rotates clockwise (CW), moving away from the bundle. The flagella are hooked to molecular rotary motors of nanometric diameter able to make transitions between CCW and CW rotations that last up to one hundredth of a second. At the same time, flagella move or rotate the bacteria's body microscopically during lapses that range between a tenth and ten seconds. We assume that the transitions between CCW and CW rotations occur solely by fluctuations of CheY- P molarity in the presence of two threshold values, and that a veto rule selects the run or tumble motions. We present Langevin equations for the CheY-P molarity in the vicinity of each molecular motor. This model allows to obtain the run- or tumble-time distribution as a linear combination of decreasing exponentials that is a function of the steady molarity of CheY-P in the neighbourhood of the molecular motor, which fits experimental data. In turn, if the internal signaling system is unstimulated, we show that the runtime distributions reach power-law behaviour, a characteristic of self-organized systems, in some time range and, afterwards, exponential cutoff. -

Scaffolding Student's Conceptions of Proportional Size and Scale
AC 2008-2115: SCAFFOLDING STUDENT’S CONCEPTIONS OF PROPORTIONAL SIZE AND SCALE COGNITION WITH ANALOGIES AND METAPHORS Alejandra Magana , Network for Computational Nanotechnology Purdue University Alejandra Magana is a Ph.D. student in Engineering Education at Purdue University. She holds a M.S. Ed. in Educational Technology from Purdue University and a M.S. in E-commerce from ITESM in Mexico City. She is currently working for the Network for Computational Nanotechnology at Purdue University as a Research Assistant and as an Instructional Designer. Sean Brophy, Purdue University Sean Brophy is an Assistant Professor in Engineering Education at Purdue University. He holds a Ph.D. in Education and Human Development (Technology in Education) from Vanderbilt University and a M.S. in Computer Science (Artificial Intelligence) from DePaul University. Timothy Newby, Purdue University Timothy J. Newby is a Professor in Curriculum and Instruction at Purdue University. He holds a Ph.D. in Instructional Psychology form the Brigham Young University. Page 13.1063.1 Page © American Society for Engineering Education, 2008 Scaffolding Student’s Conceptions of Proportional Size and Scale Cognition with Analogies and Metaphors Abstract The American Association for the Advancement of Science identifies scale as one of the four powerful common themes that transcend disciplinary boundaries and levels. Engineering is one of these disciplines that requires a strong spatial ability involving scale, as well as the ability to reason proportionally when using scale models. In addition, advancing nanosciences is opening new opportunities for engineers to pursue opportunities for designing nanotechnologies. However, today’s middle school students do not demonstrate an adequate understanding of concepts of scale and size on the micro and the nano level. -

Biofilm Growth in Porous Media: Derivation of a Macroscopic Model from the Physics … 171
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Repositorio da Universidade da Coruña BIOFILM GROWTH IN POROUS MEDIA: DERIVATION OF A MACROSCOPIC MODEL FROM THE PHYSICS … 171 Biofilm growth in porous media: derivation of a macroscopic model from the physics at the pore scale via homogenization A. Philippe1,2, C. Geindreau1, P. Séchet2 and J. Martins3 1 Lab. 3S, CNRS-UJF-INPG, BP 53X, 38041 Grenoble cedex 9, France 2 LEGI, CNRS-UJF-INPG, BP 53X, 38041 Grenoble cedex 9, France 3 LTHE, CNRS-UJF-INPG, BP 53X, 38041 Grenoble cedex 9, France ABSTRACT. This paper concerns the modelling of biofilm in porous media. A macroscopic model (at the Darcy’s scale) is derived from the physics at the pore scale by using an upscaling technique, namely the homogenisation method of multiple scale expansions. The domain of validity of this macroscopic description depends on the order of magnitude of different dimensionless numbers arising from the description at the microscopic scale. We show that at the macroscopic scale (i) the flow is described by the classical Darcy's law (ii) the mass balance of the fluid includes a source term due to the biomass growth (iii) the transport of substrate is describe by a diffusion-advection-reaction equation (iv) the macroscopic biomass balance is given by a diffusion-reaction relation. The macroscopic reactive term is similar to the microscopic one. The homogenization process also shows that all the effective coefficients (permeability, effective diffusion tensors) arising in the macroscopic description depends on the biomass fraction. -

Nanotechnologies
КАЗАНСКИЙ ФЕДЕРАЛЬНЫЙ УНИВЕРСИТЕТ ИНСТИТУТ МЕЖДУНАРОДНЫХ ОТНОШЕНИЙ, ИСТОРИИ И ВОСТОКОВЕДЕНИЯ Кафедра иностранных языков для физико-математического направления и информационных технологий С.М. ПЕРЕТОЧКИНА, Г.М. ГАТИЯТУЛЛИНА, Е.В. МАРТЫНОВА NANOTECHNOLOGIES Учебное пособие по английскому языку КАЗАНЬ 2018 УДК 53 ББК 22.3 П27 Печатается по рекомендации учебно-методической комиссии Института международных отношений, истории и востоковедения КФУ (протокол № 9 от 21 июня 2018 года) Рецензенты: кандидат педагогических наук, доцент кафедры иностранных языков для физико-математического направления и информационных технологий КФУ Х.Ф. Макаев; кандидат филологических наук, доцент кафедры иностранных языков КНИТУ-КАИ им. А.Н. Туполева Е.В. Мусина Переточкина С.М. П27 Nanotechnologies: учеб. пособие по английскому языку / С.М. Переточкина, Г.М. Гатиятуллина, Е.В. Мартынова. – Казань: Изд-во Казан. ун-та, 2018. – 146 с. Данное пособие предназначено для студентов Института физики, обу- чающихся по направлению 28.03.01 «Нанотехнологии и микросистемная техника». Пособие может быть использовано как для аудиторной работы, так и для самостоятельной работы студентов. УДК 53 ББК 22.3 © Переточкина С.М., Гатиятуллина Г.М., Мартынова Е.В., 2018 © Издательство Казанского университета, 2018 Предисловие Настоящее учебное пособие предназначено для занятий со студентами 2 курса Института физики Казанского (Приволжского) федерального универ- ситета), обучающихся по направлению 28.03.01 «Нанотехнологии и микро- системная техника». Учебное пособие разработано для развития навыков чтения текстов по специальности, создания у студентов необходимого в профессиональной дея- тельности лексического запаса, отработки навыков перевода специальных и научных текстов, а также навыков устной и письменной речи. При отборе текстового материала в качестве основного критерия слу- жила информативная ценность текстов и их соответствие специальности сту- дентов. Большинство текстов пособия взято из оригинальной английской и американской литературы. -

How Do Small Things Make a Big Difference?
October 2014 How do small things make a big difference? Microbes, ecology, and the tree of life Lesson 3: What are microbes? I. Overview This lesson defines the term “microbe” and introduces students to the diversity of microbes. Students first watch an engaging video that reviews some elements of the previous lessons about the place of microbes in the tree of life while making connections to what is to come as the unit continues to explore the different roles and ubiquity of microbes. The second activity scaffolds students’ concept of scale as they work with units and conversions to become more familiar with the microscopic scale. This concept of scale is then applied in the third activity where students make a “microbe mural”. To create the mural, students produce scaled up versions of different microbes and discuss aspects of microbial diversity such as their size, where they live, and their metabolic properties. Connections to the driving question This lesson provides students with essential background to answer the unit driving question. Students learn about how small microbes are (a defining characteristic), and how ubiquitous and diverse they are. Connections to previous lessons The previous two lessons tell the story of the tree of life and the role of microbes in that story. This lesson draws on this and shifts the focus entirely to the microbes they have been working to classify. Students discuss not only the domain-level classification of the microbes but also their size, where they live, and what they do. II. Standards National Science Education Standards Use technology and mathematics to improve investigations and communications. -

The Second Law of Thermodynamics at the Microscopic Scale Thibaut Josset
The Second Law of Thermodynamics at the Microscopic Scale Thibaut Josset To cite this version: Thibaut Josset. The Second Law of Thermodynamics at the Microscopic Scale. Foundations of Physics, Springer Verlag, 2017, 47 (9), pp.1185-1190. 10.1007/s10701-017-0104-5. hal-01662847 HAL Id: hal-01662847 https://hal.archives-ouvertes.fr/hal-01662847 Submitted on 24 Apr 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. The second law of thermodynamics at the microscopic scale Thibaut Josset Aix Marseille Univ, Universit´ede Toulon, CNRS, CPT, Marseille, France (Dated: February 27, 2017) In quantum statistical mechanics, equilibrium states have been shown to be the typical states for a system that is entangled with its environment, suggesting a possible identification between ther- modynamic and von Neumann entropies. In this paper, we investigate how the relaxation toward equilibrium is made possible through interactions that do not lead to significant exchange of energy, and argue for the validity of the second law of thermodynamics at the microscopic scale. The vast majority of phenomena we witness in every In other words, in the framework of typicality, the actual day life are irreversible. -

Last Revised 7/24/00 Microscopic Origins of Irreversible Macroscopic
Last revised 7/24/00 Microscopic Origins of Irreversible Macroscopic Behavior: An Overview* by Joel L. Lebowitz Departments of Mathematics and Physics Rutgers University Piscataway, New Jersey [email protected] Abstract Time-asymmetric behavior as embodied in the second law of thermodynamics is ob- served in individual macroscopic systems. It can be understood as arising naturally from time-symmetric microscopic laws when account is taken of a) the great disparity between microscopic and macroscopic scales, b) initial conditions, and c) the fact that what we ob- serve is “typical” behavior of real systems—not all imaginable ones. This is in accord with the ideas of Maxwell, Thomson, and Boltzmann and their natural quantum extensions. Common alternate explanations, such as those based on equating irreversible macroscopic behavior with the ergodic or mixing properties of probability distributions (ensembles) already present for chaotic dynamical systems having only a few degrees of freedom or on the impossibility of having a truly isolated system, are either unnecessary, misguided or misleading. Specific features of macroscopic evolution, such as the diffusion equation, do however depend on the dynamical instability (deterministic chaos) of trajectories of isolated macroscopic systems.Time-asymmetric behavior as embodied in the second law of * This article summarizes some of the material in [1]; see also Appendix A. 1 thermodynamics is observed in individual macroscopic systems. It can be understood as arising naturally from time-symmetric microscopic laws when account is taken of a) the great disparity between microscopic and macroscopic scales, b) initial conditions, and c) the fact that what we observe is “typical” behavior of real systems—not all imaginable ones. -

Clay Characterisation from Nanoscopic to Microscopic Resolution
Radioactive Waste Management NEA/RWM/CLAYCLUB(2013)1 May 2013 www.oecd-nea.org Clay characterisation from nanoscopic to microscopic resolution NEA CLAY CLUB Workshop Proceedings Karlsruhe, Germany 6-8 September 2011 NEA Unclassified NEA/RWM/CLAYCLUB(2013)1 Organisation de Coopération et de Développement Économiques Organisation for Economic Co-operation and Development 28-May-2013 ___________________________________________________________________________________________ _____________ English - Or. English NUCLEAR ENERGY AGENCY RADIOACTIVE WASTE MANAGEMENT COMMITTEE Unclassified NEA/RWM/CLAYCLUB(2013)1 Cancels & replaces the same document of 24 May 2013 Integration Group for the Safety Case (IGSC) CLAY CHARACTERISATION FROM NANOSCOPIC TO MICROSCOPIC RESOLUTION NEA CLAY CLUB Workshop Proceedings Karlsruhe, Germany 6-8 September 2011 Update of the title of the document on the official OLIS cover page. For any additional information, please contact Mr. Hiroomi AOKI at [email protected]. English JT03340363 Complete document available on OLIS in its original format - This document and any map included herein are without prejudice to the status of or sovereignty over any territory, to the delimitation of Or. English international frontiers and boundaries and to the name of any territory, city or area. NEA/RWM/CLAYCLUB(2013)1 FOREWORD A wide spectrum of argillaceous media are being considered in Nuclear Energy Agency (NEA) mem- ber countries as potential host rocks for the final, safe disposal of radioactive waste, and/or as major constituent of repository systems in which wastes will be emplaced. In this context, the NEA estab- lished the Working Group on the "Characterisation, the Understanding and the Performance of Argil- laceous Rocks as Repository Host Formations" in 1990, informally known as the "Clay Club". -

Unit 1. Matter
Physics and Chemistry 2º ESO Unit 1. Matter Unit 1. Matter From the moment you wake up in the morning you don´t stop seeing all sort of objects: chairs, glasses, furniture, bottles full of water, coins… All objects are made of matter. Matter is everything that has mass and takes up space . A substance is a specific type of matter that is different from any other (water, paper, wood, iron …). Substances forms materials which can be used to make objects. How can you know from what substance an object is made? How can you differentiate between substances, and then identify them? In this chapter you will see what are the characteristic properties of the substances , and why they are usefull to identify them. You will also learn about the model that we use to explain the structure of matter (what would you see if you watch a substance with a powerfull microscope). For example, a solid like copper sulfate...do you think that you would see something similar to a block, like the one on the image? Learning about this model is going to be very usefull to interpret the properties of matter, and you will be able to relate their characteristics in macroscopic scale (experimental) with the ones in microscopic scale (of particles). 1. The properties of substances There are a number of qualitative properties that are characteristic of any substance and that can be useful to identify them. Some examples of these properties are odor (although there are a lot of odorless substances), color (but there are a lot of them which have the same color!), or flavor (but you´d better not taste some of them, they can be very dangerous!).