A Phase III Randomized Controlled Trial of Short-Course Radiotherapy with Or
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SSHM Proceedings 1948-49
tcbe ~cotti~b ~ocietJ2 of tbe l)i~tor)2 of ~ebicine (Founded April, 1948.) REPORT OF PROCEEDINGS SESSION 1948.. 49. (!Cue .$cotttsb ,$ocictp of tbe JlistOiP of jRel:1icine. President Dr. DOUGLAS GUTHRIE. V iee-P l'esiden ts Mr W. 1. STUART. Profe3sor G. B. FLEMING (Glasgow) Hon. Secretary Dr. H. P. TAIT. Hon. TreaSUI'er Dr. W. A. ALEXANDER. Council Sir HENRY WADE. Dr. W. D. D. SMALL. Brig.-Gen. SUTTON. Professor CAMPBELL (Aberdeen). Dr. JOHN RITCHIE. Dr. HENRY GIBSON (Dundee). Dr. WILKIE MILLAR. Professor CHAS. M'NEIL. Mr A. L. GOODALL (Glasgow). The Senior President, Royal Medical Society. E ~bt 8tottisb ~otietp of tbe j!)istorp of ~ebicine. For many years it had been felt that there was a need for a Society in Scotland primarily devoted to the study of the History of Medicine and its allied Sciences. Such a Society came into being on 23rd April 1948, when a well attended and representative gathering of medical men and other interested persons from all over Scotland met in the Hall of the Royal College of Surgeons of Edinburgh. It was then agreed to constitute the Society and to call it "The Scottish Society of the History of Medicine." A Constitution was drawn up and Office-Bearers for the ensuing year were elected. From this beginning the Society has grown steadily and now has a membership of some hundred persons. After the business of this Preliminary 1\1 eeting had been carried through, the Medical Superintendent of the Royal Infirmary of Dundee, Dr. Henry J. C. -

Japanese Delegation of Athletics Team for Doha,Qatar 2019 27 SEP-06 OCT
Japanese Delegation of Athletics Team For Doha,Qatar 2019 27 SEP-06 OCT IAAF World Championships in Athletics-Doha,Qatar 第 17 回 IAAF 世界陸上競技選手権大会 ( カタ ー ル ・ドー ハ ) ❶ Hirooki ARAI(L)& Kai KOBAYASHI(R) Play Back London 2017 [プレイバック・ロンドン大会2017] 前回の2017年ロンドン大会では男子50kmW勢が躍動。 荒井広宙が2位、小林快が3位とダブル表彰台に上り、 丸尾知司も5位に入りました。また、男子4×100mR も3位に入り、世界選手権では初のメダルを獲得。また、 サニブラウン アブデルハキームは男子100mで準決勝、 200mでは決勝に進出(7位)。日本はメダル3、入賞2 の成績を収めました。 ❷ Men’s 4×100m Relay ❸ Satoshi MARUO ❹ Abdul Hakim SANIBROWN Japanese Medalists & Prizewinners in London 2017 Silver Athlete Record Men 50kmW Hirooki ARAI ❶Left 3.41.17 Bronze Men 50kmW Kai KOBAYASHI ❶Right 3.41.19 S.TADA,S.IIZUKA, Men 4×100mR 38.04 Y.KIRYU,K.FUJIMITSU❷ 5th Men 50kmW Satoshi MARUO❸ 3.43.03 7th Men 200m Abdul Hakim SANIBROWN❹ 20.63 02 Message[メッセージ] thletes aiming at the top of the world will be gathering in the blazing city Doha. The IAAF World Athletics Championships Doha 2019 is a great stageA for you to challenge the “power and skill” of the world, and it has an important meaning as a prelude to 2020 Tokyo Olympic Games which is quickly approaching. Expand your athletic ability you have gained through competition experiences and years of hard training here in Doha and make a huge step towards the grand stage. Along with your athletic ability, human quality is also very important. Athletics is an individual sport except for relays, but it is necessary to have Team JAPAN awareness. The consciousness of competing as a team will also enhance your human quality, and that rise helps to improve individual competitiveness. For athletes and staff, I ask you to unite by respecting each other, and have the spirit of “One for All, All for One”. -

Wednesday 12Th December 2018 Retiral of Norma Watson from FDCA Committee Purchase of Photograph of Dundee Polic
Friends of Dundee City Archives newsletter Winter 2018 17 Cake and Chat: Wednesday The Great War: Dundee & The 12th December 2018 Home Front We have arranged a Christmas Cake and Chat to be held in Committee Room 1 between 2pm and 4 pm. This room is accessed from 14 City Square and is in the corridor leading to the Archives office. We look forward to seeing you. Linda Nicoll’s book was launched at the Wighton Centre on 8 November. The book, Retiral of Norma Watson which is only available from FDCA Committee from the City Archives, is selling It was with regret that the members of the well. If you or any of FDCA Committee received Norma’s decision your family or friends to retire. Norma served as Hon. Treasurer would like a copy, from 2011 until 2016 and remained on the please either call at the Committee for a further two years. archives during office hours or apply by post, The Volunteers miss her cheery banter on email ([email protected]) or Wednesday mornings. We all wish her well in telephone (01382 434494) to Dundee City her many interests. Archives. The book costs £9.99 plus post and Purchase of Photograph of packing. Dundee Police Pipe Band This Poppy has all the names on the Dundee FDCA purchased a black and white Roll of Honour on its petals. It was part of a photograph of Dundee Police Pipe Band in the display at Fintry Primary School in 1930s for the archives. The photograph was commemoration of the 100th anniversary of taken on the steps leading to what was then the the Armistice. -

Can High-Frequency Ultrasound Predict Metastatic Lymph Nodes in Patients with Invasive Breast Cancer?
This is a repository copy of Can high-frequency ultrasound predict metastatic lymph nodes in patients with invasive breast cancer? . White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/1045/ Article: Clough, G.R., Truscott, J. and Haigh, L.I.G. (2004) Can high-frequency ultrasound predict metastatic lymph nodes in patients with invasive breast cancer? Breast Cancer Research, 6 (Suppl ). p. 18. ISSN 1465-542X https://doi.org/10.1186/bcr837 Reuse See Attached Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Available online http://breast-cancer-research.com/supplements/6/S1 Abstracts from Symposium Mammographicum 2004 Edinburgh International Conference Centre, 19–20 July 2004 1 Developing new treatments for breast cancer D Lane University of Dundee, UK and Cyclacel Ltd, UK Breast Cancer Res 2004, 6(Suppl 1):P1 (DOI 10.1186/bcr820) Twenty-five years after its first description the p53 protein has A key regulator is Mdm2, an E3 ubiquitin ligase, that binds and been shown to play a key role in both cancer and ageing. The p53 ubiquitinates p53 and directs its degradation via the proteosome. protein is activated by many different stress pathways, including Small potent peptides that can block the p53 Mdm2 interaction oncogene action and DNA damage. The elucidation of the p53 and activate the p53 response have been described. -

The Dundee Directory
^mhtlltx, BMtiMf |)rmte, $ ^d\hkkxf 10 CASTLE 5TKEET, DUNDEE, MANUFACTURES Ledgers, Journals, Day-Books, and all kinds of ACCOUNT-BOOKS, to any pattern, and of the best material and workmanship. Special attention is given to this department, and, as Ruling, Printing, Binding, and Paging, are all done on his Premises, Merchants, Manufacturers, Bankers, and others, can depend upon having their Business Books made with accuracy, despatch, and economy. An excellent assortment of BOOKS in the various departments of Literature always on hand. Any work not in Stock can be pro- cured on the shortest notice. Books, Pamphlets, Bills, Circulars, Prices- Current, and every description of LETTER -PRESS PRINTING, executed with neatness and despatch. Check Books and Cards numbered consecutively by the Paging Machine. \^ Lithographic and Copperplate Printing. PIANOFORTES by the most approved makers. MUSICAL INSTRUMENTS,— viz.: Violins, Flutes, Cornopeans, Con- certinas, Flutinas, Accordions, &c. &c. Bands furnished with every description of Brass and Wood Instruments at the most rea- sonable rates. A Large Stock of Pianoforte and other MUSIC always on hand, and parcels of the newest publications received weekly from London. BOOKBINDING in all its branches. Bibles, Testaments, Prayer-Books, and Church Services, in great variety of plain and elegant bindings. Periodicals and Newspapers regularly supplied, and all the leading Magazines and Serials lent out to read. Customhouse Entries and Forms, Wholesale and Retail. Writing Paper and Envelopes stamped with crest or initials. Stamping Presses furnished, with Devices to any pattern. AGENT FOR Price's Patent FIRE and THIEF-PROOF SAFES, The best and cheapest Safeguards in the World. -

Dundee City Archives: Subject Index
Dundee City Archives: Subject Index This subject index provides a brief overview of the collections held at Dundee City Archives. The index is sorted by topic, and in some cases sub-topics. The page index on the next page gives a brief overview of the subjects included. The document only lists the collections that have been deposited at Dundee City Archives. Therefore it does not list records that are part of the Dundee City Council Archive or any of its predecessors, including: School Records Licensing Records Burial Records Minutes Planning Records Reports Poorhouse Records Other council Records If you are interested in records that would have been created by the council or one of its predecessors, please get in contact with us to find out what we hold. This list is update regularly, but new accessions may not be included. For up to date information please contact us. In most cases the description that appears in the list is a general description of the collection. It does not list individual items in the collections. We may hold further related items in collections that have not been catalogued. For further information please contact us. Please note that some records may be closed due to restrictions such as data protection. Other records may not be accessible as they are too fragile or damaged. Please contact us for further information or check access restrictions. How do I use this index? The page index on the next page gives a list of subjects covered. Click on the subject in the page index to be taken to main body of the subject index. -

HEEL and TOE ONLINE the Official
HEEL AND TOE ONLINE The official organ of the Victorian Race Walking Club 2014/2015 Number 47 25 August 2015 VRWC Preferred Supplier of Shoes, clothes and sporting accessories. Address: RUNNERS WORLD, 598 High Street, East Kew, Victoria (Melways 45 G4) Telephone: 03 9817 3503 Hours: Monday to Friday: 9:30am to 5:30pm Saturday: 9:00am to 3:00pm Website: http://www.runnersworld.com.au Facebook: http://www.facebook.com/pages/Runners-World/235649459888840 WALKER OF THE WEEK A quick word before going to my Walker of the Week. Since I am in China at the moment, I am locked out of quite a few of the resources from which I put together my weekly newsletter – facebook, twitter, dropbox, all google facilities and various other normally inoffensive websites, not to mention my own gmail account. So if your results aren't there or I haven't responded to your email, it may be that I was just not able to pick them up. Email me via [email protected] and I can rectify in next week's newsletter. And now onto business! This weeks' Walker of the Week goes to 23 year old Australian representative Dane Bird-Smith. Racing in the IAAF World Championship 20km race on Sunday morning, he overcome a mid-race bingle and an upset stomach to finish eighth, confirming his status as the next big thing in Australian walking. Dane was among a group of several walkers who crashed over a flower bed in the middle of the course at the 12km mark but he was able to regroup to claim eighth spot in a time of 1:21:37, three places better than on his world titles debut two years ago in Moscow. -

Supplement to the Edinburgh Gazette of April 21, 1936. 365
SUPPLEMENT TO THE EDINBURGH GAZETTE OF APRIL 21, 1936. 365 Clyde Navigation Trust. Institution of Engineers and Shipbuilders in Dundee Chamber of Commerce. Scotland. Dundee Harbour Trustees. Institution of Municipal and County Engineers Edinburgh Chamber of Commerce and Manu- —Scottish District. factures. International Order of Good Templars—Grand Edinburgh Company of Merchants. Lodge of Scotland. Glasgow Chamber of Commerce and Manufac- Kinross County and Burgh, Representatives of tures. all the Public Bodies in. Glasgow Fishmongers Company. Knights of St. Columba. Glasgow Royal Exchange. Lanarkshire Association for Nurses. Glasgow Stock Exchange Association. Leifch Chamber of Commerce. National Federation of Property Owners and Leith Corporation of Trinity House. Factors of Scotland. Leith Dock Commissioners. Order of the Eastern Star. Merchants' House of Glasgow. Orkney and Zetland, Inhabitants of. Scottish Co-operative Wholesale Society. Perth County and City Royal Infirmary. 'Scottish National Building Trades Federation Perth Guildry Incorporation. (Employers). Princess Louise Scottish Hospital for Limbless Scottish Trade Protection Society. Sailors and Soldiers. Selkirk Merchants Company. Protestant Action Society, Edinburgh. Stirling Gas Light Company. Royal Caledonian Hunt. Trades House of Glasgow. Royal Caledonian Schools, Bushey. Royal Edinburgh Hospital for Incurables. Royal Edinburgh Hospital for Mental and Other Bodies. Nervous Disorders. Aberdeen Royal Infirmary. Royal Edinburgh Hospital for Sick Children. Aberlour Orphanage. Royal Hospital for Sick Children, Glasgow. Association of Registrars of Scotland. Royal Northern Club. Botanical Society of Edinburgh. Royal Philosophical Society of Glasgow. Brechin Guildry Incorporation. Royal Samaritan Hospital for Women, Glas- British Legion (Scotland): — gow. Royal Scots Fusiliers' (Edinburgh) Club. National Executive Council. Baltasound Branch. Royal Scottish Academy. Royal Scottish Corporation. Fife and Kinross Area Council. -
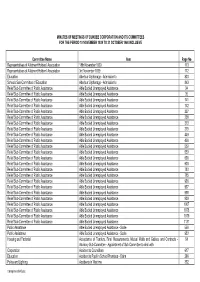
MINUTES of MEETINGS of DUNDEE CORPORATION and ITS COMMITTEES for the PERIOD 10 NOVEMBER 1939 to 31 OCTOBER 1940 INCLUSIVE Commit
MINUTES OF MEETINGS OF DUNDEE CORPORATION AND ITS COMMITTEES FOR THE PERIOD 10 NOVEMBER 1939 TO 31 OCTOBER 1940 INCLUSIVE Committee Name Item Page No Representatives of Allotment Holders' Association 18th November 1939 113 Representatives of Allotment Holders' Association 3rd November 1939 112 Education Aberlour Orphanage - Admission to 803 Schools Sub-Committee of Education Aberlour Orphanage - Admission to 843 Relief Sub-Committee of Public Assistance Able Bodied Unemployed Assistance 34 Relief Sub-Committee of Public Assistance Able Bodied Unemployed Assistance 35 Relief Sub-Committee of Public Assistance Able Bodied Unemployed Assistance 141 Relief Sub-Committee of Public Assistance Able Bodied Unemployed Assistance 142 Relief Sub-Committee of Public Assistance Able Bodied Unemployed Assistance 227 Relief Sub-Committee of Public Assistance Able Bodied Unemployed Assistance 228 Relief Sub-Committee of Public Assistance Able Bodied Unemployed Assistance 313 Relief Sub-Committee of Public Assistance Able Bodied Unemployed Assistance 315 Relief Sub-Committee of Public Assistance Able Bodied Unemployed Assistance 449 Relief Sub-Committee of Public Assistance Able Bodied Unemployed Assistance 450 Relief Sub-Committee of Public Assistance Able Bodied Unemployed Assistance 552 Relief Sub-Committee of Public Assistance Able Bodied Unemployed Assistance 553 Relief Sub-Committee of Public Assistance Able Bodied Unemployed Assistance 638 Relief Sub-Committee of Public Assistance Able Bodied Unemployed Assistance 639 Relief Sub-Committee of Public -

Angus and Mearns Directory and Almanac, 1846
21 DAYS ALLOWED FOR READING THIS BOOK. Overdue Books Charged at Ip per Day. FORFAR PUBLIC LIBRARY IL©CAIL C©iLILECirD©IN ANGUS - CULTURAL SERVICES lllllllllillllllllllllllllllillllllllllllllllllllllllllllll Presented ^m . - 01:91^ CUStPI .^HE isms AND MSARNS ' DIRECTORY FOR 18^6 couni Digitized by tlie Internet Arcliive in 2010 witli funding from National Library of Scotland http://www.archive.org/details/angusmearnsdirec1846unse - - 'ir- AC'-.< u —1 >- GQ h- D >- Q. a^ LU 1*- <f G. O (^ O < CD i 1 Q. o U. ALEX MAC HABDY THE ANGUS AND MEAENS DIRECTORY FOR 1846, CONTAINING IN ADDITION TO THE WHOLE OP THE LISTS CONNECTED WITH THE COUNTIES OP FORFAR AND KINCARDINE, AND THE BURGHS OP DUNDEE, MONTROSE, ARBROATH, FORFAR, KIRRIEMUIR, STONEHAVEN, &c, ALPHABETICAL LISTS 'of the inhabitants op MONTROSE, ARBROATH, FORFAR, BRECBIN, AND KIRRIEMUIR; TOGETHEK WITH A LIST OF VESSELS REGISTERED AT THE PORTS OF MONTROSE, ARBROATH, DUNDEE, PERTH, ABERDEEN AND STONEHAVEN. MONTROSE PREPARED AND PUBLISHED BY JAMUI^ \VATT, STANDARD OFFICE, AND SOIiD BY ALL THE BOOKSELLERS IN THE TWO COUNTIES. EDINBURGH: BLACKWOOD & SON, AND OLIVER &c BOYD, PRINTED AT THE MONTROSE STANDARD 0FFIC5 CONTENTS. Page. Page Arbroath Dfrectory— Dissenting Bodies 178 Alphabetical List of Names 84 Dundee DtRECTORY— Banks, Public Offices, &c. 99 Banks, Public Offices, &c. 117 Burgh Funds . 102 Burgh Funds .... 122 Biiri^h Court 104 Banking Companies (Local) 126 128 Bible Society . • 105 Burgh Court .... Coaches, Carriers, &c. 100 Building Company, Joint-Stock 131 Comraerciiil Associations . 106 Coaches 11« Cliarities . , 106 Carriers 119 Educational Institutions . 104 Consols for Foreign States 121 Fire and Life Insurance Agents 101 Cemetery Company 124 Friendly Societies . -

Race Walking Judges in Olympic Games 1908-2012
IAAF Race Walking Committee Members 1935-2015 The Council of the IAAF at a meeting In Berlin 1935 decided to appoint a special commission to study the rules of walking as well as organization questions in different countries with regard to walking. At the same time the answers to the circular letter sent out by the IAAF office concerning the Olympic walking races were to be studied by the commission which met in Paris 1.2.1936. 1935 Committee for 'Walking': Jean Genet, (FRA), Chairman. 1947 Walking Commission: Fred Blackmore, (GBR) President Ernest Holt, (GBR) Secretary Francis Guilleux (FRA) Giorgio Oberweger (ITA). B. Kopal (CZE). A. Larsen (NOR). Eric G. Linde (SWE) 1951 Walking Commission. Fred Blackmore, (GBR) President Ernest Holt, (GBR) Secretary Francis Guilleux (FRA) Bela Fehervari (HUN) Bruno Zauli, (ITA) A. M. Hagen (NOR) Eric G. Linde (SWE) Armando Libotte (SUI) 1952 Walking Commission. Fred Blackmore, (GBR) President Donald Pain, (GBR) Hon. Secretary, Francis Guilleux (FRA) Bela Fehervari (HUN) Giorgio Oberweger (ITA). A. M. Hagen (NOR) I. lonescu (ROM) Eric G. Linde (SWE) Armando Libotte (SUI) Nicolai Kalinin (USSR) 1956 Walking Commission Giorgio Oberweger, (ITA) President Donald Pain, (GBR) Hon. Secretary, Hubert Sulak (TCH) Francis Guilleux (FRA) Harold Whitlock (GBR) Bela Fehervari (HUN) A. M. Hagen (NOR) I. lonescu (ROM) Eric G. Linde (SWE) Armando Libotte (SUI) Nicolai Kalinin (USSR) 1960 Walking Commission Giorgio Oberweger, (ITA) President Donald Pain, (GBR) Hon. Secretary, Bela Fehervari (HUN) Francis Guilleux (FRA) A. M. Hagen (NOR) I. lonescu (ROM) Eric G. Linde (SWE) Armando Libotte (SUI) Hubert Sulak (CZE) Peter Stepanenko (U.S.S.R) Harold Whitlock (GBR) 1964 WALKING COMMISSION Giorgio Oberweger, (ITA) President Donald Pain, (GBR) Hon. -

DDSR Document Scanning
IDh) r ~rnttisl1 ~nriety (@f iqr t;istnry nf :!Iebiriue (Founded April, 1948) REPORT OF PROCEEDINGS SESSION 2000-2001 and 2001-2002 UJ4e .§cottt54 ~n(t.ety of t4e 1A"ii5tnry of :atebictnr OFFICE BEARERS (2000-2001) (2001-2002) President Dr J FORRESTER Dr DJ WRIGHT Vice-Presidents DrHTSWAN DrJFORRESTER DrDJWRIGHT Dr B ASHWORTH HOt! Secretm:v DrAR BUTLER DrARBUTLER Hon Treasurer Dr J SIMPSON Dr J SIMPSON Hon Auditor Dr RUFUS ROSS Dr RUFUS ROSS Hot! Editor Dr DJ WRIGHT Dr DJ WRIGHT Council Dr B ASHWORTH Dr DAVID BOYD Mrs AILSA BLAIR Mr J CHALMERS Mr J CHALMERS Dr JAMES GRAY MRS E GEISSLER Mrs M HAGGART Dr JAMES GRAY Prof RI McCALLUM Or E JELLINEK OrK MILLS Mrs M HAGGART Mr I MILNE Prof RI McCALLUM Or RUFUS ROSS ProfTH PENNINGTON Dr MJ WILLIAMS IDqc ~cotttsq ~octcty ®ftqc 1!;tstory of mrbtctnc (Founded April, 1948) Report ofProceedings CONTENTS a) The Health of Dundee Jute Workers Dr Anne Hargreaves b) The National Health Service- The Plan for Scotland 9 Dr Morrice McCrae c) Dr lan MacQueen and the Aberdeen Typhoid Outbreak of 1964 16 Dr Lesley Diack & Dr David Smith d) Thomas Winterbottom and the Welfare of Mariners 24 Dr Stuart Menzies e) Our Unique NHS: Past, Present and Future? 30 Sir Alexander Macara f) Some Caithness Doctors and Diseases 38 Dr David Boyd g) Sir John Franklin and Polar Medicine 43 Mr Ken Mills h) A Minister's Brilliant Progeny 45 Mr Roy Miller i) A Decided Novelty for the British Army 49 Dr John Cule j) Penrose, Eugenics and Scotland 51 Dr David Watt k) Alfred Nobel and the Medical Prizes 59 Dr B,yan Ashworth 1) Transplantation ofTeeth 61 Dr Henry Noble m) The Russell Family 65 Dr Ernest Jellinek SESSION 2000-2001 and 2001-2002 The Scottish Society of the History of Medicine REPORT OF PROCEEDINGS SESSION 2000-2001 THE FIFTY SECOND ANNUAL GENERAL MEETING The Fifty Second Annual General of the Society was held at the Verdant Works, Dundee on the 4'h November 2000.