A Comprehensive Review of Portosystemic Collaterals in Cirrhosis: Historical Aspects, Anatomy, and Classifications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Anatomy of the Rectum and Anal Canal
BASIC SCIENCE identify the rectosigmoid junction with confidence at operation. The anatomy of the rectum The rectosigmoid junction usually lies approximately 6 cm below the level of the sacral promontory. Approached from the distal and anal canal end, however, as when performing a rigid or flexible sigmoid- oscopy, the rectosigmoid junction is seen to be 14e18 cm from Vishy Mahadevan the anal verge, and 18 cm is usually taken as the measurement for audit purposes. The rectum in the adult measures 10e14 cm in length. Abstract Diseases of the rectum and anal canal, both benign and malignant, Relationship of the peritoneum to the rectum account for a very large part of colorectal surgical practice in the UK. Unlike the transverse colon and sigmoid colon, the rectum lacks This article emphasizes the surgically-relevant aspects of the anatomy a mesentery (Figure 1). The posterior aspect of the rectum is thus of the rectum and anal canal. entirely free of a peritoneal covering. In this respect the rectum resembles the ascending and descending segments of the colon, Keywords Anal cushions; inferior hypogastric plexus; internal and and all of these segments may be therefore be spoken of as external anal sphincters; lymphatic drainage of rectum and anal canal; retroperitoneal. The precise relationship of the peritoneum to the mesorectum; perineum; rectal blood supply rectum is as follows: the upper third of the rectum is covered by peritoneum on its anterior and lateral surfaces; the middle third of the rectum is covered by peritoneum only on its anterior 1 The rectum is the direct continuation of the sigmoid colon and surface while the lower third of the rectum is below the level of commences in front of the body of the third sacral vertebra. -

Splenic Artery Embolization for the Treatment of Gastric Variceal Bleeding Secondary to Splenic Vein Thrombosis Complicated by Necrotizing Pancreatitis: Report of a Case
Hindawi Publishing Corporation Case Reports in Medicine Volume 2016, Article ID 1585926, 6 pages http://dx.doi.org/10.1155/2016/1585926 Case Report Splenic Artery Embolization for the Treatment of Gastric Variceal Bleeding Secondary to Splenic Vein Thrombosis Complicated by Necrotizing Pancreatitis: Report of a Case Hee Joon Kim, Eun Kyu Park, Young Hoe Hur, Yang Seok Koh, and Chol Kyoon Cho Department of Surgery, Chonnam National University Medical School, Gwangju, Republic of Korea Correspondence should be addressed to Chol Kyoon Cho; [email protected] Received 11 August 2016; Accepted 1 November 2016 Academic Editor: Omer Faruk Dogan Copyright © 2016 Hee Joon Kim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Splenic vein thrombosis is a relatively common finding in pancreatitis. Gastric variceal bleeding is a life-threatening complication of splenic vein thrombosis, resulting from increased blood flow to short gastric vein. Traditionally, splenectomy is considered the treatment of choice. However, surgery in necrotizing pancreatitis is dangerous, because of severe inflammation, adhesion, and bleeding tendency. In the Warshaw operation, gastric variceal bleeding is rare, even though splenic vein is resected. Because the splenic artery is also resected, blood flow to short gastric vein is not increased problematically. Herein, we report a case of gastric variceal bleeding secondary to splenic vein thrombosis complicated by necrotizing pancreatitis successfully treated with splenic artery embolization. Splenic artery embolization could be the best treatment option for gastric variceal bleeding when splenectomy is difficult such as in case associated with severe acute pancreatitis or associated with severe adhesion or in patients withhigh operation risk. -

Portal Vein Stenting for Jejunal Variceal Bleeding After Recurrence of Pancreatic Adenocarcinoma: a Case Report and Review of the Literature
Case Report Portal Vein Stenting for Jejunal Variceal Bleeding after Recurrence of Pancreatic Adenocarcinoma: A Case Report and Review of the Literature 1) Department of Radiology, Kyushu University Beppu Hospital, Japan 2) Department of Surgery, Kyushu University Beppu Hospital, Japan 3) Department of Clinical Radiology, Graduate School of Medical Science, Kyushu University, Japan Seiichiro Takao1), Masakazu Hirakawa1), Kazuki Takeishi2), Yushi Motomura1), Katsumi Sakamoto1), Hajime Otsu2), Yusuke Yonemura2), Koshi Mimori2), Kousei Ishigami3) Abstract A 73-year-old woman with portal vein stenosis caused by tumor recurrence after pancreatoduodenectomy was treated with stent placement without embolization of the jejunal varix. Anticoagulation therapy using heparin followed by rivaroxaban was administered after the procedure. She continued to receive systemic chemotherapy as an outpatient. Neither restenosis nor stent thrombosis was observed after 7 months. Based on the presented case and literature review, portal vein stenting is an effective treatment option for jejunal variceal bleeding caused by malignant portal venous stricture after pancreaticoduodenectomy. Antithrombotic therapy following portal venous stenting is required to prevent stent thrombosis in the majority of cases, al- though it has a risk of inducing recurrent variceal bleeding. Adjunctive jejunal variceal embolization can pos- sibly be omitted in selected cases to obtain sufficient portal-SMV flow reconstruction. Key words: Portal vein, Constriction, Stents (Interventional Radiology 2021; 6: 44-50) port describes a case of successful stenting for a patient Introduction with portal venous stenosis and bleeding from jejunal varices after pancreatoduodenectomy, along with the relevant Recurrent pancreatic cancer can cause portal venous literature. stenosis, resulting in symptoms of portal hypertension, such as hemorrhagic tendencies and liver dysfunction. -

Anatomical Planes in Rectal Cancer Surgery
DOI: 10.4274/tjcd.galenos.2019.2019-10-2 Turk J Colorectal Dis 2019;29:165-170 REVIEW Anatomical Planes in Rectal Cancer Surgery Rektum Kanser Cerrahisinde Anatomik Planlar Halil İbrahim Açar, Mehmet Ayhan Kuzu Ankara University Faculty of Medicine, Department of General Surgery, Ankara, Turkey ABSTRACT This review outlines important anatomical landmarks not only for rectal cancer surgery but also for pelvic exentration. Keywords: Anorectal anatomy, pelvic anatomy, surgical anatomy of rectum ÖZ Pelvis anatomisini derleme halinde özetleyen bu makale rektum kanser cerrahisi ve pelvik ezantrasyon için önemli topografik noktaları gözden geçirmektedir. Anahtar Kelimeler: Anorektal anatomi, pelvik anatomi, rektumun cerrahi anatomisi Introduction Surgical Anatomy of the Rectum The rectum extends from the promontory to the anal canal Pelvic Anatomy and is approximately 12-15 cm long. It fills the sacral It is essential to know the pelvic anatomy because of the concavity and ends with an anal canal 2-3 cm anteroinferior intestinal and urogenital complications that may develop to the tip of the coccyx. The rectum contains three folds in after the surgical procedures applied to the pelvic region. the coronal plane laterally. The upper and lower are convex The pelvis, encircled by bone tissue, is surrounded by the to the right, and the middle is convex to the left. The middle main vessels, ureters, and autonomic nerves. Success in the fold is aligned with the peritoneal reflection. Intraluminal surgical treatment of pelvic organs is only possible with a projections of the lower boundaries of these folds are known as Houston’s valves. Unlike the sigmoid colon, taenia, good knowledge of the embryological development of the epiploic appendices, and haustra are absent in the rectum. -

Prep for Practical II
Images for Practical II BSC 2086L "Endocrine" A A B C A. Hypothalamus B. Pineal Gland (Body) C. Pituitary Gland "Endocrine" 1.Thyroid 2.Adrenal Gland 3.Pancreas "The Pancreas" "The Adrenal Glands" "The Ovary" "The Testes" Erythrocyte Neutrophil Eosinophil Basophil Lymphocyte Monocyte Platelet Figure 29-3 Photomicrograph of a human blood smear stained with Wright’s stain (765). Eosinophil Lymphocyte Monocyte Platelets Neutrophils Erythrocytes "Blood Typing" "Heart Coronal" 1.Right Atrium 3 4 2.Superior Vena Cava 5 2 3.Aortic Arch 6 4.Pulmonary Trunk 1 5.Left Atrium 12 9 6.Bicuspid Valve 10 7.Interventricular Septum 11 8.Apex of The Heart 9. Chordae tendineae 10.Papillary Muscle 7 11.Tricuspid Valve 12. Fossa Ovalis "Heart Coronal Section" Coronal Section of the Heart to show valves 1. Bicuspid 2. Pulmonary Semilunar 3. Tricuspid 4. Aortic Semilunar 5. Left Ventricle 6. Right Ventricle "Heart Coronal" 1.Pulmonary trunk 2.Right Atrium 3.Tricuspid Valve 4.Pulmonary Semilunar Valve 5.Myocardium 6.Interventricular Septum 7.Trabeculae Carneae 8.Papillary Muscle 9.Chordae Tendineae 10.Bicuspid Valve "Heart Anterior" 1. Brachiocephalic Artery 2. Left Common Carotid Artery 3. Ligamentum Arteriosum 4. Left Coronary Artery 5. Circumflex Artery 6. Great Cardiac Vein 7. Myocardium 8. Apex of The Heart 9. Pericardium (Visceral) 10. Right Coronary Artery 11. Auricle of Right Atrium 12. Pulmonary Trunk 13. Superior Vena Cava 14. Aortic Arch 15. Brachiocephalic vein "Heart Posterolateral" 1. Left Brachiocephalic vein 2. Right Brachiocephalic vein 3. Brachiocephalic Artery 4. Left Common Carotid Artery 5. Left Subclavian Artery 6. Aortic Arch 7. -

Venous and Lymphatic Vessels. ANATOM.UA PART 1
Lection: Venous and lymphatic vessels. ANATOM.UA PART 1 https://fipat.library.dal.ca/ta2/ Ch. 1 Anatomia generalis PART 2 – SYSTEMATA MUSCULOSKELETALIA Ch. 2 Ossa Ch. 3 Juncturae Ch. 4 Musculi PART 3 – SYSTEMATA VISCERALIA Ch. 5 Systema digestorium Ch. 6 Systema respiratorium Ch. 7 Cavitas thoracis Ch. 8 Systema urinarium Ch. 9 Systemata genitalia Ch. 10 Cavitas abdominopelvica PART 4 – SYSTEMATA INTEGRANTIA I Ch. 11 Glandulae endocrinae Ch. 12 Systema cardiovasculare Ch. 13 Organa lymphoidea PART 5 – SYSTEMATA INTEGRANTIA II Ch. 14 Systema nervosum Ch. 15 Organa sensuum Ch. 16 Integumentum commune ANATOM.UA ANATOM.UA Cardiovascular system (systema cardiovasculare) consists of the heart and the tubes, that are used for transporting the liquid with special functions – the blood or lymph, that are necessary for supplying the cells with nutritional substances and the oxygen. ANATOM.UA 5 Veins Veins are blood vessels that bring blood back to theheart. All veins carry deoxygenatedblood with the exception of thepulmonary veins and umbilical veins There are two types of veins: Superficial veins: close to the surface of thebody NO corresponding arteries Deep veins: found deeper in the body With corresponding arteries Veins of the systemiccirculation: Superior and inferior vena cava with their tributaries Veins of the portal circulation: Portal vein ANATOM.UA Superior Vena Cava Formed by the union of the right and left Brachiocephalic veins. Brachiocephalic veins are formed by the union of internal jugular and subclavianveins. Drains venous blood from: Head &neck Thoracic wall Upper limbs It Passes downward and enter the rightatrium. Receives azygos vein on the posterior aspect just before it enters theheart. -

Corona Mortis: the Abnormal Obturator Vessels in Filipino Cadavers
ORIGINAL ARTICLE Corona Mortis: the Abnormal Obturator Vessels in Filipino Cadavers Imelda A. Luna Department of Anatomy, College of Medicine, University of the Philippines Manila ABSTRACT Objectives. This is a descriptive study to determine the origin of abnormal obturator arteries, the drainage of abnormal obturator veins, and if any anastomoses exist between these abnormal vessels in Filipino cadavers. Methods. A total of 54 cadaver halves, 50 dissected by UP medical students and 4 by UP Dentistry students were included in this survey. Results. Results showed the abnormal obturator arteries arising from the inferior epigastric arteries in 7 halves (12.96%) and the abnormal communicating veins draining into the inferior epigastric or external iliac veins in 16 (29.62%). There were also arterial anastomoses in 5 (9.25%) with the inferior epigastric artery, and venous anastomoses in 16 (29.62%) with the inferior epigastric or external iliac veins. Bilateral abnormalities were noted in a total 6 cadavers, 3 with both arterial and venous, and the remaining 3 with only venous anastomoses. Conclusion. It is important to be aware of the presence of these abnormalities that if found during surgery, must first be ligated to avoid intraoperative bleeding complications. Key Words: obturator vessels, abnormal, corona mortis INtroDUCTION The main artery to the pelvic region is the internal iliac artery (IIA) with two exceptions: the ovarian/testicular artery arises directly from the aorta and the superior rectal artery from the inferior mesenteric artery (IMA). The internal iliac or hypogastric artery is one of the most variable arterial systems of the human body, its parietal branches, particularly the obturator artery (OBA) accounts for most of its variability. -

CASE REPORT Spinal Cord Infarction Following Endoscopic Variceal Ligation
Spinal Cord (2008) 46, 241–242 & 2008 International Spinal Cord Society All rights reserved 1362-4393/08 $30.00 www.nature.com/sc CASE REPORT Spinal cord infarction following endoscopic variceal ligation K Tofuku, H Koga, T Yamamoto, K Yone and S Komiya Department of Orthopaedic Surgery, Kagoshima Graduate School of Medical and Dental Sciences, Kagoshima, Japan Study design: A case report of spinal cord infarction following endoscopic variceal ligation. Objectives: To describe an exceedingly rare case of spinal cord infarction following endoscopic variceal ligation. Setting: Department of Orthopaedic Surgery, Kagoshima, Japan. Methods: A 75-year-old woman with cirrhosis caused by hepatitis C virus, who was admitted to our hospital for the treatment of esophageal varices, experienced numbness of the hands and lower extremities bilaterally following an endoscopic variceal ligation procedure. Sensory and motor dysfunction below C6 level progressed rapidly, resulting in inability to move the lower extremities the following day. Magnetic resonance imaging of the spine revealed abnormal spinal cord signal on T2-weighted images from approximately C6 through T5 levels, which was diagnosed as spinal cord infarction. Results: The patient underwent hyperbaric oxygen treatment. Her symptoms and signs related to spinal cord infarction gradually remitted, and nearly complete disappearance of neurological deficits was noted within 3 months after the start of treatment. Conclusion: We speculate that the pathogenesis of the present case may have involved congestion of the abdominal–epidural–spinal cord venous network owing to ligation of esophageal varices and increased thoracoabdominal cavity pressure. Spinal Cord (2008) 46, 241–242; doi:10.1038/sj.sc.3102092; published online 19 June 2007 Keywords: endoscopic variceal ligation; hyperbaric oxygen; spinal cord infarction Introduction The spectrum of etiologies of spinal cord infarction is as On physical examination, her right upper extremity diverse as that for cerebral infarction. -

Autologous Unilateral Breast Reconstruction with Venous Supercharged IMAP-Flaps: a Step by Step Guide of the Split Breast Technique
Journal of Clinical Medicine Article Autologous Unilateral Breast Reconstruction with Venous Supercharged IMAP-Flaps: A Step by Step Guide of the Split Breast Technique , Kathrin Bachleitner * y, Laurenz Weitgasser y, Amro Amr and Thomas Schoeller Department of Hand, Breast, and Reconstructive Microsurgery, Marienhospital Stuttgart, 70199 Stuttgart, Germany; [email protected] (L.W.); [email protected] (A.A.); [email protected] (T.S.) * Correspondence: [email protected]; Tel.: +49-711-6489-7202 Both authors contributed equally to this work. y Received: 13 August 2020; Accepted: 18 September 2020; Published: 20 September 2020 Abstract: Various techniques for breast reconstruction ranging from reconstruction with implants to free tissue transfer, with the disadvantage of either carrying a foreign body or dealing with donor site morbidity, have been described. In patients who had a unilateral mastectomy and offer a contralateral mamma hypertrophy a breast reconstruction can be performed with the excess tissue from the hypertrophic side using the split breast technique. Here a local internal mammary artery perforator (IMAP) flap of the hypertrophic breast can be used for reconstruction avoiding the downsides of implants or a microsurgical reconstruction and simultaneously reducing the enlarged donor breast in order to achieve symmetry. Methods: Between April 2010 and February 2019 the split breast technique was performed in five patients after mastectomy due to breast cancer. Operating time, length of stay, complications and the need for secondary operations were analyzed and the surgical technique including flap supercharging were described in detail. Results: All five IMAP-flaps survived and an aesthetically pleasant result could be achieved using the split breast technique. -
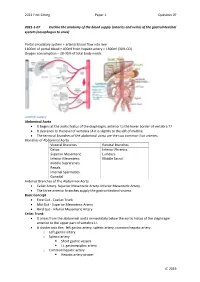
Arteries and Veins) of the Gastrointestinal System (Oesophagus to Anus)
2021 First Sitting Paper 1 Question 07 2021-1-07 Outline the anatomy of the blood supply (arteries and veins) of the gastrointestinal system (oesophagus to anus) Portal circulatory system + arterial blood flow into liver 1100ml of portal blood + 400ml from hepatic artery = 1500ml (30% CO) Oxygen consumption – 20-35% of total body needs Arterial Supply Abdominal Aorta • It begins at the aortic hiatus of the diaphragm, anterior to the lower border of vertebra T7. • It descends to the level of vertebra L4 it is slightly to the left of midline. • The terminal branches of the abdominal aorta are the two common iliac arteries. Branches of Abdominal Aorta Visceral Branches Parietal Branches Celiac. Inferior Phrenics. Superior Mesenteric. Lumbars Inferior Mesenteric. Middle Sacral. Middle Suprarenals. Renals. Internal Spermatics. Gonadal Anterior Branches of The Abdominal Aorta • Celiac Artery. Superior Mesenteric Artery. Inferior Mesenteric Artery. • The three anterior branches supply the gastrointestinal viscera. Basic Concept • Fore Gut - Coeliac Trunk • Mid Gut - Superior Mesenteric Artery • Hind Gut - Inferior Mesenteric Artery Celiac Trunk • It arises from the abdominal aorta immediately below the aortic hiatus of the diaphragm anterior to the upper part of vertebra LI. • It divides into the: left gastric artery, splenic artery, common hepatic artery. o Left gastric artery o Splenic artery ▪ Short gastric vessels ▪ Lt. gastroepiploic artery o Common hepatic artery ▪ Hepatic artery proper JC 2019 2021 First Sitting Paper 1 Question 07 • Left hepatic artery • Right hepatic artery ▪ Gastroduodenal artery • Rt. Gastroepiploic (gastro-omental) artery • Sup pancreatoduodenal artery • Supraduodenal artery Oesophagus • Cervical oesophagus - branches from inferior thyroid artery • Thoracic oesophagus - branches from bronchial arteries and aorta • Abd. -

Split Azygos Vein: a Case Report
Open Access Case Report DOI: 10.7759/cureus.13362 Split Azygos Vein: A Case Report Stefan Lachkar 1 , Joe Iwanaga 2 , Emma Newton 2 , Aaron S. Dumont 2 , R. Shane Tubbs 2 1. Anatomy, Seattle Chirdren's, Seattle, USA 2. Neurosurgery, Tulane University School of Medicine, New Orleans, USA Corresponding author: Joe Iwanaga, [email protected] Abstract The azygos venous system, which comprises the azygos, hemiazygos, and accessory hemiazygos veins, assists in blood drainage into the superior vena cava (SVC) from the thoracic cage and portions of the posterior mediastinum. Routine dissection of a fresh-frozen cadaveric specimen revealed a split azygos vein. The azygos vein branched off the inferior vena cava (IVC) at the level of the second lumbar vertebra as a single trunk and then split into two tributaries after forming a venous plexus. The right side of this system drained into the SVC and, inferiorly, the collective system drained into the IVC. Variant forms in the venous system, especially the vena cavae, are prone to dilation and tortuosity, leading to an increased likelihood of injury. Knowledge of the anatomical variations of the azygos vein is important for surgeons who use an anterior approach to the spine for diverse procedures. Categories: Anatomy Keywords: inferior vena cava, embryology, azygos vein, variation, anatomy, cadaver Introduction The inferior vena cava (IVC) is the largest vein in the human body. Its principal function is to return venous blood from the abdomen and lower extremities to the right atrium of the heart [1]. Developmental patterning of the IVC consists of three paired embryonic veins: subcardinal, supracardinal, and postcardinal. -

Portal Vein: a Review of Pathology and Normal Variants on MDCT E-Poster: EE-005
Portal vein: a review of pathology and normal variants on MDCT e-Poster: EE-005 Congress: ESGAR2016 Type: Educational Exhibit Topic: Diagnostic / Abdominal vascular imaging Authors: C. Carneiro, C. Bilreiro, C. Bahia, J. Brito; Portimao/PT MeSH: Abdomen [A01.047] Portal System [A07.231.908.670] Portal Vein [A07.231.908.670.567] Hypertension, Portal [C06.552.494] Any information contained in this pdf file is automatically generated from digital material submitted to e-Poster by third parties in the form of scientific presentations. References to any names, marks, products, or services of third parties or hypertext links to third-party sites or information are provided solely as a convenience to you and do not in any way constitute or imply ESGAR’s endorsement, sponsorship or recommendation of the third party, information, product, or service. ESGAR is not responsible for the content of these pages and does not make any representations regarding the content or accuracy of material in this file. As per copyright regulations, any unauthorised use of the material or parts thereof as well as commercial reproduction or multiple distribution by any traditional or electronically based reproduction/publication method is strictly prohibited. You agree to defend, indemnify, and hold ESGAR harmless from and against any and all claims, damages, costs, and expenses, including attorneys’ fees, arising from or related to your use of these pages. Please note: Links to movies, ppt slideshows and any other multimedia files are not available in the pdf version of presentations. www.esgar.org 1. Learning Objectives To review the embryology and anatomy of the portal venous system.