Switching Antipsychotics a Balanced Approach
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aripiprazole As an Adjunct to Anti-Depressants for Major Depressive Disorder: Clinical Effectiveness, Cost-Effectiveness, and Guidelines
TITLE: Aripiprazole as an Adjunct to Anti-Depressants for Major Depressive Disorder: Clinical Effectiveness, Cost-Effectiveness, and Guidelines DATE: 04 May 2016 RESEARCH QUESTIONS 1. What is the clinical efficacy of aripiprazole as an adjunct to antidepressants for the treatment of patients with major depressive disorder who have had an inadequate response to prior antidepressant treatments? 2. What is the cost-effectiveness of aripiprazole as an adjunct to antidepressants for the treatment of patients with major depressive disorder who have had an inadequate response to prior antidepressant treatments? 3. What are the evidence-based guidelines for the use of aripiprazole for the treatment of patients with major depressive disorder who have had an inadequate response to prior antidepressant treatments? KEY FINDINGS Three systematic reviews with meta-analyses, two randomized controlled trials, and two evidence-based guidelines were identified regarding the use of aripiprazole as an adjunct to antidepressants for the treatment of patients with major depressive disorder who have had an inadequate response to prior antidepressant treatments. METHODS A limited literature search was conducted on key resources including PubMed, Ovid PsychINFO, Ovid Embase, The Cochrane Library, University of York Centre for Reviews and Dissemination (CRD) databases, Canadian and major international health technology agencies, as well as a focused Internet search. No methodological filters were used to limit retrieval by study type. Where possible, retrieval was limited to the human population. The search was also limited to English language documents published between January 1, 2014 and April 29, 2016. Disclaimer: The Rapid Response Service is an information service for those involved in planning and providing health care in Canada. -

Download Executive Summary
Comparative Effectiveness Review Number 43 Effective Health Care Program Off-Label Use of Atypical Antipsychotics: An Update Executive Summary Background Effective Health Care Program Antipsychotics medications are approved The Effective Health Care Program by the U.S. Food and Drug Administration was initiated in 2005 to provide valid (FDA) for treatment of schizophrenia and evidence about the comparative bipolar disorder. These medications are effectiveness of different medical commonly divided into two classes, interventions. The object is to help reflecting two waves of historical consumers, health care providers, and development: the conventional others in making informed choices antipsychotics and the atypical. The among treatment alternatives. Through conventional antipsychotics served as the its Comparative Effectiveness Reviews, first successful pharmacologic treatment the program supports systematic for primary psychotic disorders such as appraisals of existing scientific schizophrenia. Having been widely used evidence regarding treatments for for decades, the conventional high-priority health conditions. It also antipsychotics also produced various side promotes and generates new scientific effects requiring additional medications, evidence by identifying gaps in which spurred the development of the existing scientific evidence and atypical antipsychotics. supporting new research. The program Currently, nine atypical antipsychotic drugs puts special emphasis on translating have been approved by FDA: aripiprazole, findings into a variety of useful asenapine, clozapine, iloperidone, formats for different stakeholders, olanzapine, paliperidone, quetiapine, including consumers. risperidone, and ziprasidone. These drugs The full report and this summary are have been used off-label (i.e., for available at www.effectivehealthcare. indications not approved by FDA) for the ahrq.gov/reports/final.cfm. treatment of various psychiatric conditions. -
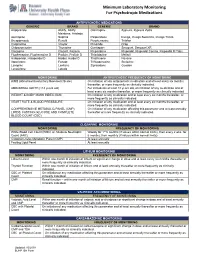
Minimum Laboratory Monitoring for Psychotropic Medications
Minimum Laboratory Monitoring For Psychotropic Medications ANTIPSYCHOTIC MEDICATIONS GENERIC BRAND GENERIC BRAND Aripiprazole Abilify, Abilify Olanzapine Zyprexa, Zyprexa Zydis Maintena, Aristada Asenapine Saphris Paliperidone Invega, Invega Sustenna, Invega Trinza Brexpiprazole Rexulti Perphenazine Trilafon Cariprazine Vraylar Pimozide Orap Chlorpromazine Thorazine Quetiapine Seroquel, Seroquel XR Clozapine Clozaril, Fazaclo Risperidone Risperdal, Risperdal Consta, Risperdal M Tabs Fluphenazine, Fluphenazine D Prolixin, Prolixin D Thioridazine Mellaril Haloperidol, Haloperidol D Haldol, Haldol D Thiothixene Navane Iloperidone Fanapt Trifluoperazine Stelazine Loxapine Loxitane Ziprasidone Geodon Lurasidone Latuda MONITORING ANTIPSYCHOTIC FREQUENCY OF MONITORING AIMS (Abnormal Involuntary Movement Scale) On initiation of any antipsychotic medication and at least every six months thereafter, or more frequently as clinically indicated. ABDOMINAL GIRTH (>18 years old) For individuals at least 18 years old, on initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. WEIGHT & BODY MASS INDEX (BMI) On initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. HEART RATE & BLOOD PRESSSURE On initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. COMPREHENSIVE METABOLIC PANEL (CMP), On initiation of any medication affecting this parameter and at least annually LIPIDS, FASTING -

Schizophrenia Care Guide
August 2015 CCHCS/DHCS Care Guide: Schizophrenia SUMMARY DECISION SUPPORT PATIENT EDUCATION/SELF MANAGEMENT GOALS ALERTS Minimize frequency and severity of psychotic episodes Suicidal ideation or gestures Encourage medication adherence Abnormal movements Manage medication side effects Delusions Monitor as clinically appropriate Neuroleptic Malignant Syndrome Danger to self or others DIAGNOSTIC CRITERIA/EVALUATION (PER DSM V) 1. Rule out delirium or other medical illnesses mimicking schizophrenia (see page 5), medications or drugs of abuse causing psychosis (see page 6), other mental illness causes of psychosis, e.g., Bipolar Mania or Depression, Major Depression, PTSD, borderline personality disorder (see page 4). Ideas in patients (even odd ideas) that we disagree with can be learned and are therefore not necessarily signs of schizophrenia. Schizophrenia is a world-wide phenomenon that can occur in cultures with widely differing ideas. 2. Diagnosis is made based on the following: (Criteria A and B must be met) A. Two of the following symptoms/signs must be present over much of at least one month (unless treated), with a significant impact on social or occupational functioning, over at least a 6-month period of time: Delusions, Hallucinations, Disorganized Speech, Negative symptoms (social withdrawal, poverty of thought, etc.), severely disorganized or catatonic behavior. B. At least one of the symptoms/signs should be Delusions, Hallucinations, or Disorganized Speech. TREATMENT OPTIONS MEDICATIONS Informed consent for psychotropic -

Current P SYCHIATRY
Current p SYCHIATRY N ew Investigators Tips to manage and prevent discontinuation syndromes Informed tapering can protect patients when you stop a medication Sriram Ramaswamy, MD Shruti Malik, MBBS, MHSA Vijay Dewan, MD Instructor, department of psychiatry Foreign medical graduate Assistant professor Creighton University Department of psychiatry Omaha, NE University of Nebraska Medical Center Omaha, NE bruptly stopping common psychotropics New insights on psychotropic A —particularly antidepressants, benzodi- drug safety and side effects azepines, or atypical antipsychotics—can trigger a discontinuation syndrome, with: This paper was among those entered in the 2005 • rebound or relapse of original symptoms Promising New Investigators competition sponsored • uncomfortable new physical and psycho- by the Neuroleptic Malignant Syndrome Information Service (NMSIS). The theme of this year’s competition logical symptoms was “New insights on psychotropic drug safety and • physiologic withdrawal at times. side effects.” To increase health professionals’ awareness of URRENT SYCHIATRY 1 C P is honored to publish this peer- the risk of these adverse effects, this article reviewed, evidence-based article on a clinically describes discontinuation syndromes associated important topic for practicing psychiatrists. with various psychotropics and offers strategies to NMSIS is dedicated to reducing morbidity and anticipate, recognize, and manage them. mortality of NMS by improving medical and psychiatric care of patients with heat-related disorders; providing -

Clinical Review, Adverse Events
Clinical Review, Adverse Events Drug: Carbamazepine NDA: 16-608, Tegretol 20-712, Carbatrol 21-710, Equetro Adverse Event: Stevens-Johnson Syndrome Reviewer: Ronald Farkas, MD, PhD Medical Reviewer, DNP, ODE I 1. Executive Summary 1.1 Background Carbamazepine (CBZ) is an anticonvulsant with FDA-approved indications in epilepsy, bipolar disorder and neuropathic pain. CBZ is associated with Stevens-Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN), closely related serious cutaneous adverse drug reactions that can be permanently disabling or fatal. Other anticonvulsants, including phenytoin, phenobarbital, and lamotrigine are also associated with SJS/TEN, as are members of a variety of other drug classes, including nonsteriodal anti-inflammatory drugs and sulfa drugs. The incidence of CBZ-associated SJS/TEN has been considered “extremely rare,” as noted in current U.S. drug labeling. However, recent publications and postmarketing data suggest that CBZ- associated SJS/TEN occurs at a much higher rate in some Asian populations, about 2.5 cases per 1,000 new exposures, and that most of this increased risk is in individuals carrying a specific human leukocyte antigen (HLA) allele, HLA-B*1502. This HLA-B allele is present in about 5- to 20% of many, but not all, Asian populations, and is also present in about 2- to 4% of South Asians/Indians. The allele is also present at a lower frequency, < 1%, in several other ethnic groups around the world (although likely due to distant Asian ancestry). About 10% of U.S. Asians carry HLA-B*1502. HLA-B*1502 is generally not present in the U.S. -

Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment for Schizophrenia in Adult Patients? Kyle J
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Student Dissertations, Theses and Papers Scholarship 2017 Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients? Kyle J. Knowles Philadelphia College of Osteopathic Medicine Follow this and additional works at: https://digitalcommons.pcom.edu/pa_systematic_reviews Part of the Psychiatry Commons Recommended Citation Knowles, Kyle J., "Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients?" (2017). PCOM Physician Assistant Studies Student Scholarship. 381. https://digitalcommons.pcom.edu/pa_systematic_reviews/381 This Selective Evidence-Based Medicine Review is brought to you for free and open access by the Student Dissertations, Theses and Papers at DigitalCommons@PCOM. It has been accepted for inclusion in PCOM Physician Assistant Studies Student Scholarship by an authorized administrator of DigitalCommons@PCOM. For more information, please contact [email protected]. Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients? Kyle J. Knowles, PA-S A SELECTIVE EVIDENCE BASED MEDICINE REVIEW In Partial Fulfillment of the Requirements For The Degree of Master of Science In Health Sciences- Physician Assistant Department of Physician Assistant Studies Philadelphia College of Osteopathic Medicine Philadelphia, Pennsylvania December 16, 2016 ABSTRACT OBJECTIVE: The objective of this selective EBM review is to determine whether or not “Is Aristada (aripiprazole lauroxil) a safe and effective treatment for schizophrenia in adult patients?” STUDY DESIGN: Review of three randomized controlled studies. All three trials were conducted between 2014 and 2015. DATA SOURCES: One randomized, controlled trial and two randomized, controlled, double- blind trials found via Cochrane Library and PubMed. -

Doxepin Exacerbates Renal Damage, Glucose Intolerance, Nonalcoholic Fatty Liver Disease, and Urinary Chromium Loss in Obese Mice
pharmaceuticals Article Doxepin Exacerbates Renal Damage, Glucose Intolerance, Nonalcoholic Fatty Liver Disease, and Urinary Chromium Loss in Obese Mice Geng-Ruei Chang 1,* , Po-Hsun Hou 2,3, Wei-Cheng Yang 4, Chao-Min Wang 1 , Pei-Shan Fan 1, Huei-Jyuan Liao 1 and To-Pang Chen 5,* 1 Department of Veterinary Medicine, National Chiayi University, 580 Xinmin Road, Chiayi 60054, Taiwan; [email protected] (C.-M.W.); [email protected] (P.-S.F.); [email protected] (H.-J.L.) 2 Department of Psychiatry, Taichung Veterans General Hospital, 1650 Taiwan Boulevard (Section 4), Taichung 40705, Taiwan; [email protected] 3 Faculty of Medicine, National Yang-Ming University, 155 Linong Street (Section 2), Taipei 11221, Taiwan 4 School of Veterinary Medicine, National Taiwan University, 1 Roosevelt Road (Section 4), Taipei 10617, Taiwan; [email protected] 5 Division of Endocrinology and Metabolism, Show Chwan Memorial Hospital, 542 Chung-Shan Road (Section 1), Changhua 50008, Taiwan * Correspondence: [email protected] (G.-R.C.); [email protected] (T.-P.C.); Tel.: +886-5-2732946 (G.-R.C.); +886-4-7256166 (T.-P.C.) Abstract: Doxepin is commonly prescribed for depression and anxiety treatment. Doxepin-related disruptions to metabolism and renal/hepatic adverse effects remain unclear; thus, the underlying mechanism of action warrants further research. Here, we investigated how doxepin affects lipid Citation: Chang, G.-R.; Hou, P.-H.; change, glucose homeostasis, chromium (Cr) distribution, renal impairment, liver damage, and fatty Yang, W.-C.; Wang, C.-M.; Fan, P.-S.; liver scores in C57BL6/J mice subjected to a high-fat diet and 5 mg/kg/day doxepin treatment for Liao, H.-J.; Chen, T.-P. -

Olanzapine-Induced Acute Pancreatitis and New Diabetes Mellitus
Open Journal of Psychiatry, 2012, 2, 110-112 OJPsych http://dx.doi.org/10.4236/ojpsych.2012.22015 Published Online April 2012 (http://www.SciRP.org/journal/ojpsych/) Case report: Olanzapine-induced acute pancreatitis and new diabetes mellitus Erik Monasterio1*, Ruchi Bhalla2, Andrew McKean3 1Department of Psychological Medicine, University of Otago, Christchurch, New Zealand 2Hammersmith and Fulham Mental Health Unit, West London Mental Health NHS Trust, London, UK 3Hillmorton Hospital, Christchurch, New Zealand Email: *[email protected] Received 29 December 2011; revised 31 January 2012; accepted 15 February 2012 ABSTRACT criteria and required application from a psychiatrist for a patient who had been trialled unsuccessfully on risperi- The aim of this case study is to review the literature done or required treatment with olanzapine short acting and report the first published case of olanzapine-in- intra-muscular injection [4]. In June 2011, significantly duced acute pancreatitis in New Zealand. A case re- cheaper generic versions of olanzapine were introduced port of acute pancreatitis with new onset diabetes and the special authority criteria for olanzapine with- mellitus secondary to olanzapine in a 42-year-old male, drawn [5]. We aim to highlight lesser known and poten- in the absence of medical risk factors is reported. tially fatal side effects through our case report. Eleven previous case reports of olanzapine induced acute-pancreatitis were identified in the literature. A 2. CASE REPORT 42-year-old male was diagnosed with acute pancreati- tis and new diabetes mellitus induced by olanzapine. Mr. X is a 42 year old man who had come into contact Although rare, pancreatitis is associated with use of with mental health services on many occasions during some atypical antipsychotic medications. -

Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanismss
Supplemental Material can be found at: /content/suppl/2020/12/18/73.1.202.DC1.html 1521-0081/73/1/202–277$35.00 https://doi.org/10.1124/pharmrev.120.000056 PHARMACOLOGICAL REVIEWS Pharmacol Rev 73:202–277, January 2021 Copyright © 2020 by The Author(s) This is an open access article distributed under the CC BY-NC Attribution 4.0 International license. ASSOCIATE EDITOR: MICHAEL NADER Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanismss Antonio Inserra, Danilo De Gregorio, and Gabriella Gobbi Neurobiological Psychiatry Unit, Department of Psychiatry, McGill University, Montreal, Quebec, Canada Abstract ...................................................................................205 Significance Statement. ..................................................................205 I. Introduction . ..............................................................................205 A. Review Outline ........................................................................205 B. Psychiatric Disorders and the Need for Novel Pharmacotherapies .......................206 C. Psychedelic Compounds as Novel Therapeutics in Psychiatry: Overview and Comparison with Current Available Treatments . .....................................206 D. Classical or Serotonergic Psychedelics versus Nonclassical Psychedelics: Definition ......208 Downloaded from E. Dissociative Anesthetics................................................................209 F. Empathogens-Entactogens . ............................................................209 -

Second-Generation Long-Acting Injectable Antipsychotics: a PRACTICAL GUIDE Understanding Each of These Medications’ Unique Properties Can Optimize Patient Care
Second-generation long-acting injectable antipsychotics: A PRACTICAL GUIDE Understanding each of these medications’ unique properties can optimize patient care Brittany L. Parmentier, PharmD, MPH, here are currently 7 FDA-approved second-generation long-acting BCPS, BCPP 1-7 Clinical Assistant Professor injectable antipsychotics (LAIAs). These LAIAs provide a Department of Pharmacy Practice Tunique dosage form that allows patients to receive an antipsy- The University of Texas at Tyler Fisch College of Pharmacy chotic without taking oral medications every day, or multiple times per Tyler, Texas day. This may be an appealing option for patients and clinicians, but Disclosure The author reports no financial relationships with any because there are several types of LAIAs available, it may be difficult to companies whose products are mentioned in this article, or with determine which LAIA characteristics are best for a given patient. manufacturers of competing products. Since the FDA approved the first second-generation LAIA, risperidone long-acting injectable (LAI),1 in 2003, 6 additional second-generation LAIAs have been approved: • aripiprazole LAI • aripiprazole lauroxil LAI • olanzapine pamoate LAI • paliperidone palmitate monthly injection • paliperidone palmitate 3-month LAI • risperidone LAI for subcutaneous (SQ) injection. When discussing medication options with patients, clinicians need to consider factors that are unique to each LAIA. In this article, I describe the similarities and differences among the second-generation LAIAs, and address common questions about these medications. A major potential benefit: Increased adherence One potential benefit of all LAIAs is increased medication adherence com- pared with oral antipsychotics. One meta-analysis of 21 randomized con- trolled trials (RCTs) that compared LAIAs with oral antipsychotics and Current Psychiatry GEORGE MATTEI/SCIENCE SOURCE GEORGE MATTEI/SCIENCE Vol. -

Efficacy and Tolerability of Aripiprazole Versus D2 Antagonists in the Early
www.nature.com/npjschz REVIEW ARTICLE OPEN Efficacy and tolerability of aripiprazole versus D2 antagonists in the early course of schizophrenia: a systematic review and meta-analysis David D. Kim1,2, Alasdair M. Barr1,2, Lulu Lian1, Jessica W. Y. Yuen1, Diane Fredrikson3, William G. Honer2,3, Allen E. Thornton2,4 and ✉ Ric M. Procyshyn 2,3 Early intervention is essential for favorable long-term outcomes in schizophrenia. However, there is limited guidance in the scientific literature on how best to choose between dopamine D2 receptor (D2R) partial agonists and D2R antagonists in early stages of schizophrenia. The aim of this meta-analysis was to directly compare D2R partial agonists with D2R antagonists for efficacy and tolerability, using randomized controlled trials (RCTs) that involved participants diagnosed with first-episode psychosis, schizophrenia, or related psychotic disorders with a duration of illness ≤5 years. Fourteen RCTs, involving 2494 patients, were included in the meta-analysis. Aripiprazole was the only identified D2R partial agonist, and was not significantly different from pooled D2R antagonists for overall symptom reduction or all-cause discontinuation. However, aripiprazole was more favorable than pooled D2R antagonists for depressive symptoms, prolactin levels, and triglyceride levels. Specifically, aripiprazole was more favorable than paliperidone for triglyceride levels and more favorable than risperidone and olanzapine, but less favorable than ziprasidone, for weight gain. In addition, aripiprazole was less favorable for akathisia compared with second-generation D2R 1234567890():,; antagonists, in particular olanzapine and quetiapine, and less favorable for discontinuation due to inefficacy than risperidone. Lastly, aripiprazole was more favorable than haloperidol for various efficacy and tolerability outcomes.