Code Lists Page 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Developmental Genetics of Hemoglobin Synthesis in Chironomus Darrel Starr English Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1968 The developmental genetics of hemoglobin synthesis in Chironomus Darrel Starr English Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Genetics Commons Recommended Citation English, Darrel Starr, "The developmental genetics of hemoglobin synthesis in Chironomus " (1968). Retrospective Theses and Dissertations. 3660. https://lib.dr.iastate.edu/rtd/3660 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 6 8-14,785 ENGLISH, Barrel Starr, 1936- THE DEVELOPMENTAL GENETICS OF HEMOGLOBIN SYNTHESIS IN CHIRONOMUS. Iowa State University, Ph.D., 1968 Biology- Genetics University Microfilms, Inc., Ann Arbor, Michigan THE DEVELOPMENTAL GENETICS OF HEMOGLOBIN SYNTHESIS IN CHIRONOMUS by Darrel Starr English A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Genetics Approved: Signature was redacted for privacy. In Charge of MajdA Work Signature was redacted for privacy. Head ^ Major Department Signature was redacted for privacy. -

Enzymatic Encoding Methods for Efficient Synthesis Of
(19) TZZ__T (11) EP 1 957 644 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C12N 15/10 (2006.01) C12Q 1/68 (2006.01) 01.12.2010 Bulletin 2010/48 C40B 40/06 (2006.01) C40B 50/06 (2006.01) (21) Application number: 06818144.5 (86) International application number: PCT/DK2006/000685 (22) Date of filing: 01.12.2006 (87) International publication number: WO 2007/062664 (07.06.2007 Gazette 2007/23) (54) ENZYMATIC ENCODING METHODS FOR EFFICIENT SYNTHESIS OF LARGE LIBRARIES ENZYMVERMITTELNDE KODIERUNGSMETHODEN FÜR EINE EFFIZIENTE SYNTHESE VON GROSSEN BIBLIOTHEKEN PROCEDES DE CODAGE ENZYMATIQUE DESTINES A LA SYNTHESE EFFICACE DE BIBLIOTHEQUES IMPORTANTES (84) Designated Contracting States: • GOLDBECH, Anne AT BE BG CH CY CZ DE DK EE ES FI FR GB GR DK-2200 Copenhagen N (DK) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • DE LEON, Daen SK TR DK-2300 Copenhagen S (DK) Designated Extension States: • KALDOR, Ditte Kievsmose AL BA HR MK RS DK-2880 Bagsvaerd (DK) • SLØK, Frank Abilgaard (30) Priority: 01.12.2005 DK 200501704 DK-3450 Allerød (DK) 02.12.2005 US 741490 P • HUSEMOEN, Birgitte Nystrup DK-2500 Valby (DK) (43) Date of publication of application: • DOLBERG, Johannes 20.08.2008 Bulletin 2008/34 DK-1674 Copenhagen V (DK) • JENSEN, Kim Birkebæk (73) Proprietor: Nuevolution A/S DK-2610 Rødovre (DK) 2100 Copenhagen 0 (DK) • PETERSEN, Lene DK-2100 Copenhagen Ø (DK) (72) Inventors: • NØRREGAARD-MADSEN, Mads • FRANCH, Thomas DK-3460 Birkerød (DK) DK-3070 Snekkersten (DK) • GODSKESEN, -

PROTEOMIC ANALYSIS of HUMAN URINARY EXOSOMES. Patricia
ABSTRACT Title of Document: PROTEOMIC ANALYSIS OF HUMAN URINARY EXOSOMES. Patricia Amalia Gonzales Mancilla, Ph.D., 2009 Directed By: Associate Professor Nam Sun Wang, Department of Chemical and Biomolecular Engineering Exosomes originate as the internal vesicles of multivesicular bodies (MVBs) in cells. These small vesicles (40-100 nm) have been shown to be secreted by most cell types throughout the body. In the kidney, urinary exosomes are released to the urine by fusion of the outer membrane of the MVBs with the apical plasma membrane of renal tubular epithelia. Exosomes contain apical membrane and cytosolic proteins and can be isolated using differential centrifugation. The analysis of urinary exosomes provides a non- invasive means of acquiring information about the physiological or pathophysiological state of renal cells. The overall objective of this research was to develop methods and knowledge infrastructure for urinary proteomics. We proposed to conduct a proteomic analysis of human urinary exosomes. The first objective was to profile the proteome of human urinary exosomes using liquid chromatography-tandem spectrometry (LC- MS/MS) and specialized software for identification of peptide sequences from fragmentation spectra. We unambiguously identified 1132 proteins. In addition, the phosphoproteome of human urinary exosomes was profiled using the neutral loss scanning acquisition mode of LC-MS/MS. The phosphoproteomic profiling identified 19 phosphorylation sites corresponding to 14 phosphoproteins. The second objective was to analyze urinary exosomes samples isolated from patients with genetic mutations. Polyclonal antibodies were generated to recognize epitopes on the gene products of these genetic mutations, NKCC2 and MRP4. The potential usefulness of urinary exosome analysis was demonstrated using the well-defined renal tubulopathy, Bartter syndrome type I and using the single nucleotide polymorphism in the ABCC4 gene. -

8.5 X12.5 Doublelines.P65
Cambridge University Press 978-0-521-87519-6 - Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management, Second Edition Edited by Martin H. Steinberg, Bernard G. Forget, Douglas R. Higgs and David J. Weatherall Index More information anti-inflammatory therapy, 762–763 thalassemia-related complications, 779 sulfasalazine, nuclear factor (NF)-kB, 762 transplant-related complications, 778–779 targeting ET-1, 762–763 S-linked haplotypes, African/Indo-European, anti-oxidant therapy targeting erythrocyte, 638–640 765–766 burst forming unit-erythroid (BFU-E), 10, 29 deferiprone, 765 oral glutamine, 765 calcium-activated potassium channel (Gardos oral N-acetyl-cysteine, 765–766 pathway), 167–168 anti-oxidant therapy targeting vascular, 763–765 capillary electrophoresis, 660 Index Apo A-I mimetics, 764 capillary IEF, 660 NO, 763–764 carbon monoxide poisoning, 613–616 statins, 764 clinical features, 615 xanthine oxidase inhibitors, 764–765 diagnosis, treatment, 615–616 anti-thrombotic therapy epidemiology, 613–614 -thalassemia, 761–762 cardiac, arterial abnormalities, 151 sickle cell disease, 761–762 cardiac abnormalities, ATRX syndrome, 305 aortagonad-mesonephros (AGM), 6 cardiovascular disease, 652 Apo A-I mimetics, 764 cation content, cellular hydration, 164–172 apoptosis, vasculature permeability, 193–194 calcium-activated potassium channel, 167–168 assays, assay systems, 7, 142 cation permeability alterations, 166–167 ATMDS. See ␣ thalassemia-myelodysplastic cell calcium, calcium pump activity, 167 syndromes cell sodium, -

Newborn Screening Result for Bart's Hemoglobin
NEWBORN SCREENING RESULT FOR BART’S HEMOGLOBIN Physician’s information sheet developed by the Nebraska Newborn Screening Program with review by James Harper, MD, Pediatric Hematologist with UNMC Follow-up program, and member of the Nebraska Newborn Screening Advisory Committee. BACKGROUND The alpha thalassemias result from the loss of alpha globin genes. There are normally four genes for alpha globin production so that the loss of one to four genes is possible. The lack of one gene causes alpha thalassemia 2 (silent carrier) with no clinically detectable problems but may cause small amounts of hemoglobin Barts to be present in newborn blood samples. Alpha thalassemia trait (Alpha thalassemia 1) results from loss of two genes and causes a mild microcytic anemia which may resemble iron deficiency anemia. The loss of three genes causes hemoglobin H diseases which is a moderately severe form of thalassemia. The lack of all four genes causes hydrops fetalis and is usually fatal in utero. In general, only the loss of one or two genes is seen in African Americans. Individuals from Southeast Asia and the Mediterranean may have all four types of alpha thalassemia. The percentage of hemoglobin Barts in the blood sample may indicate the number of alpha genes that have been lost. However, the percentage of hemoglobin Barts is not directly measurable with the current methodology used by the newborn screening laboratory. Only the presence of Barts hemoglobin in relation to fetal and adult hemoglobin, and variants S, C, D and E can be detected. RECOMMENDED WORK UP In addition to the standard newborn hemoglobinopathy confirmation (hemoglobin electrophoresis), to separate those patients with alpha thalassemia silent carrier from the patients with alpha thalassemia trait, we recommend that these babies have the following labs drawn at their 6 month well baby check: CBC with retic count, ferritin, and a hemoglobin electropheresis. -

LOINC Top 2000++ Lab Observations V1.6 (PDF)
LOINC Mapper's Guide to Top 2000++ US Lab Tests v1.6 June 2017 Page 1 of 112 B C EFGH I P LOINC # Long Common Name Class Rank Example Example Comments System Override UCUM UCUM Adjusted 1 Display 2 General Guidance 1) Ask your test kit and instrument manufacturer(s) and referral labs about which LOINC codes are relevant for their products. Increasingly, test kit and instrument manufacturers are requesting LOINC codes for their new test. Some of the larger manufacturers have mapped their routine tests done on to LOINC codes. Check with these in vitro diagnostic companies for the LOINC codes relevant for their tests. In addition, the largest referral laboratories in the US have mapped their high- to medium-volume tests to LOINC. Getting the LOINC mappings from either of these sources will save you time. 2) When mapping, search against the LOINC common test list. In RELMA and on search.loinc.org you can set the search parameters to only look at the common tests. Work through the mapping by lab section. Realize that LOINC does not encompass terms that may be used in your lab system for internal accounting or “diagnostic” variables that are provided as indicators that might be used to trigger a follow up test, but are not supposed to be reported to the ordering provider because the results are not reliable enough. Blood cell counters usually report such indicators. 3) Obtain a master list of tests for mapping. RELMA has a function that will convert a large set of HL7 result (ORU) messages into a database that carries the name of the order, the units of measure, and sample data that can be the source of frequency statistics for deciding which terms to tackle first. -

Cloud-Clone 16-17
Cloud-Clone - 2016-17 Catalog Description Pack Size Supplier Rupee(RS) ACB028Hu CLIA Kit for Anti-Albumin Antibody (AAA) 96T Cloud-Clone 74750 AEA044Hu ELISA Kit for Anti-Growth Hormone Antibody (Anti-GHAb) 96T Cloud-Clone 74750 AEA255Hu ELISA Kit for Anti-Apolipoprotein Antibodies (AAHA) 96T Cloud-Clone 74750 AEA417Hu ELISA Kit for Anti-Proteolipid Protein 1, Myelin Antibody (Anti-PLP1) 96T Cloud-Clone 74750 AEA421Hu ELISA Kit for Anti-Myelin Oligodendrocyte Glycoprotein Antibody (Anti- 96T Cloud-Clone 74750 MOG) AEA465Hu ELISA Kit for Anti-Sperm Antibody (AsAb) 96T Cloud-Clone 74750 AEA539Hu ELISA Kit for Anti-Myelin Basic Protein Antibody (Anti-MBP) 96T Cloud-Clone 71250 AEA546Hu ELISA Kit for Anti-IgA Antibody 96T Cloud-Clone 71250 AEA601Hu ELISA Kit for Anti-Myeloperoxidase Antibody (Anti-MPO) 96T Cloud-Clone 71250 AEA747Hu ELISA Kit for Anti-Complement 1q Antibody (Anti-C1q) 96T Cloud-Clone 74750 AEA821Hu ELISA Kit for Anti-C Reactive Protein Antibody (Anti-CRP) 96T Cloud-Clone 74750 AEA895Hu ELISA Kit for Anti-Insulin Receptor Antibody (AIRA) 96T Cloud-Clone 74750 AEB028Hu ELISA Kit for Anti-Albumin Antibody (AAA) 96T Cloud-Clone 71250 AEB264Hu ELISA Kit for Insulin Autoantibody (IAA) 96T Cloud-Clone 74750 AEB480Hu ELISA Kit for Anti-Mannose Binding Lectin Antibody (Anti-MBL) 96T Cloud-Clone 88575 AED245Hu ELISA Kit for Anti-Glutamic Acid Decarboxylase Antibodies (Anti-GAD) 96T Cloud-Clone 71250 AEK505Hu ELISA Kit for Anti-Heparin/Platelet Factor 4 Antibodies (Anti-HPF4) 96T Cloud-Clone 71250 CCA005Hu CLIA Kit for Angiotensin II -

Characterization of Aminopeptidase in the Free-Living Nematode Panagrellus Redivivus: Subcellular Distribution and Possible Role in Neuropeptide Metabolism E
Journal of Nematology 39(2):153–160. 2007. © The Society of Nematologists 2007. Characterization of Aminopeptidase in the Free-living Nematode Panagrellus redivivus: Subcellular Distribution and Possible Role in Neuropeptide Metabolism E. P. Masler Abstract: Aminopeptidase was detected in homogenates of the free-living nematode Panagrellus redivivus with the aminoacyl substrate L-alanine-4-nitroanilide. Subcellular distribution of activity was 80% soluble and 20% membrane-associated. Aminopep- tidases in the two fractions differed in affinity for Ala-4-NA, with Km’s of 0.65 mM (soluble) and 2.90 mM (membrane). Specific activities (units/mg) at pH 7.8, 27°C were 9.10 (soluble) and 14.30 (membrane). Each enzyme was competitively inhibited by amastatin (90% at 100 µM inhibitor, IC50 = 3.7 µM) and inhibited by puromycin (30% at 500 µM) and 1,10-phenanthroline (IC50’s:; 148 µM, soluble; 89 µM, membrane). Activity was restored by Zn++, with maximum recoveries of 50% (soluble) and 90% (mem- ∼ brane), each at 23 µM ZnCl2. Estimated molecular masses for each were 150 kDa. FMRFamide-like neuropeptides behaved as competitive inhibitors. Modification of the N-terminal F of FMRFamide weakened inhibition by 95%, suggesting that the N-terminus is essential for binding to the enzyme. Two nematode FMRFamides, APKPFIRFa and RNKFEFIRFa, were the most potent tested. This is the first biochemical characterization of aminopeptidase in a free-living nematode other than Caenorhabditis elegans and demon- strates the high selectivity of the P. redivivus enzymes for neuropeptide substrates. Key words: FMRFamide-like peptide, inhibitor; membrane, metallopeptidase, neuropeptide, protease Nematodes, like other eukaryotic organisms, depend reproduction (Day and Maule, 1999; Maule et al., 2002; upon proteolytic enzymes for the regulation of essential Rogers et al., 2003). -

Hematology Notes Blood/ Hematology Danil Hammoudi.MD
Hematology notes Blood/ Hematology Danil Hammoudi.MD HTTP://Sinoemedicalassociation.or/AP2/ Page | 1 Blood is a connective tissue whose matrix is fluid. It is composed of: 1. red corpuscles, 2. white cells, 3. platelets, 4. blood plasma. It is transported throughout the body within blood vessels. • Blood is sometimes considered to be a fluid connective tissue because of the mesenchymal origin of its cells and a low ratio of cells to liquid intercellular substance, the blood plasma. • In human adults about 5 liter of blood contribute 7-8 % to the body weight of the individual. • The contribution of red blood cells (erythrocytes) to the total volume of the blood (haematocrit) is about 43%. • Erythrocytes are the dominant (99%) but not the only type of cells in the blood. • We also find leukocytes and, in addition, blood platelets. Erythrocytes, leukocytes and blood platelets are also being referred to as the formed elements of the blood. • Erythrocytes and blood platelets perform their functions exclusively in the blood stream. • In contrast, leukocytes reside only temporarily in the blood. • Leukocytes can leave the blood stream through the walls of capillaries and venules and enter either connective or lymphoid tissues. Hematology notes Page | 2 Hematology notes Page | 3 Blood facts • Approximately 8% of an adult's body weight is made up of blood. • Females have around 4-5 litres, while males have around 5-6 litres. This difference is mainly due to the differences in body size between men and women. • Its mean temperature is 38 degrees Celcius. • It has a pH of 7.35-7.45, making it slightly basic (less than 7 is considered acidic). -

WO 2017/070364 Al 27 April 2017 (27.04.2017) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/070364 Al 27 April 2017 (27.04.2017) P O P C T (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 39/395 (2006.01) C07K 16/18 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, C07K 16/00 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, PCT/US20 16/057942 MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (22) International Filing Date: OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 20 October 2016 (20.10.201 6) SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (25) Filing Language: English ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 62/244,655 2 1 October 2015 (21. 10.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: QOOLABS, INC. [US/US]; 4186 Sorrento TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, Valley Blvd., Suite D/E, San Diego, CA 92121 (US). -
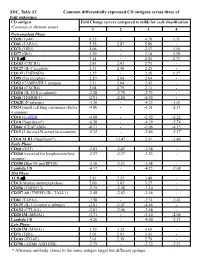
SDC, Table S1. Common Differentially Expressed CD Antigens
SDC, Table S1. Common differentially expressed CD antigens across three of four outcomes CD antigen Fold Change (severe compared to mild) for each classification (Common or alternate name) 1 2 3 4 Pretransplant Phase CD28 (Tp44) 6.32 - 4.79 3.71 CD81 (TAPA1) 5.58 2.87 5.86 - CD71 (TfR1) 4.06 - 2.57 3.16 CD77 (Gb3) 3.29 - 3.18 3.29 TCR ab 3.14 - 2.01 2.73 CD183 (CXCR3) 2.43 2.93 2.71 - CD127 (IL-7 receptor) 2.31 2.48 2.25 - CD137 (TNFRSF9) 2.27 - 2.35 2.27 CD95 (Fas receptor) 2.20 2.04 2.64 - CD52 (CAMPATH-1 antigen) 2.11 2.04 2.43 - CD184 (CXCR4) 2.04 2.79 2.11 - CD210 (IL-10 R a subunit) -2.28 -2.79 -2.73 - CD40 (TNFRSF5) -2.91 -2.20 -4.92 - CD62E (E-selectin) -3.36 - -4.23 -3.01 CD24 (small cell lung carcinoma cluster -4.00 - -3.51 -2.33 4 antigen) CD16 (FcγRIII) -4.08 - -2.92 -2.22 CD10 (Neprilysin*) -6.28 - -4.29 -5.74 CD66c (CEACAM6) -8.51 - -5.06 -6.15 CD15 (3-fucosyl-N-acetyl-lactosamine) -9.32 - -5.86 -5.17 CD10 ALB1 (Neprilysin*) - 12.47 2.51 -3.46 Early Phase CD60 (GD3) -2.03 -2.43 -2.58 - CD260 (reserved for lymphotoxin beta -2.57 -2.27 -3.23 - receptor) CD180 (Bgp-95 and RP105) -3.10 -2.33 -3.48 - Lambda 1/8 -4.17 - -4.23 -2.68 Mid Phase TCR ab 2.81 2.22 3.48 - CD13(Alanine aminopeptidase) 2.00 3.03 3.27 - CD256 (TNFSF13) -2.19 -2.48 -3.10 - CD257 AS (TNFSF13B / TALL1) -2.48 -2.62 -3.46 - CD81 (TAPA1) -2.58 - -2.31 -2.43 CD125 (IL-5 receptor a subunit) -2.81 -3.07 -4.66 - CD152 (CTLA-4) -2.81 -2.06 -3.48 - CD20 IM (MS4A1) -3.71 - -3.10 -2.04 Lambda 1/8 -4.26 - -4.00 -2.13 Late Phase CD20 IM (MS4A1) 3.39 3.32 4.69 - CD71 (TfR1) 2.03 2.93 3.01 - CD103 (ITGAE) 2.03 2.39 2.79 - CD79b (AGM6 AND IGB) -2.79 - -2.32 -2.23 * Alternate antibody clones to the same antigen target but different epitope. -

Arizona Hemoglobin Bart's Fact Sheet for Health Care Providers
Arizona Hemoglobin Bart’s Fact Sheet for Health Care Providers Hemoglobin Barts Your patient has been found on the Arizona Newborn Genetic Screen to have a hemoglobin electrophoresis pattern consistent with "FA Bart’s". The acronym stands for the hemoglobin species present in the baby's blood in descending order of prevalence. The F designates fetal hemoglobin (a2 y2), A denotes hemoglobin A (a2ß2) and Bart’s represents hemoglobin Bart's, a tetramer of y-globin molecules (y4). Hemoglobin Barts (y4) appears in the newborn when one or more of the 4 human a-globin genes are missing. The relative over abundance of y-globin molecules leads to y4 production and the diagnosis of Hemoglobin Barts. Alpha thalassemia is caused by deletions of the alpha globin genes on chromosome 16. Normal individuals have 4 copies of the gene with 2 on each chromosome. It is possible to lose 1 to 4 of these genes. The presence of hemoglobin Bart’s on newborn screen usually suggests that the infant is missing at least 1 alpha gene. The silent carrier: One deleted Alpha Gene Neonates and children with three functional alpha genes have a complete or nearly completely silent phenotype. The red cell indices are normal and remain so for life. When only one a gene is non- functional, the hemoglobin Barts percentage is usually 1-2% in the newborn, and is not detectable when the fetal hemoglobin synthesis stops at 6 months of age. As the newborn matures, the red cells can rarely exhibit a reduced MCV, MCH, but will show normal HBA2 and F levels if the hemoglobin electrophoresis is repeated.