African Vultures Don't Follow Migratory Herds: Scavenger Habitat
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

How Large Herbivores Subsidize Aquatic Food Webs in African
COMMENTARY Howlargeherbivoressubsidizeaquaticfoodwebsin African savannas COMMENTARY Robert M. Pringlea,1 Mass migration—the periodic, synchronized movement in the Kenyan portion of the Mara almost every year from of large numbers of animals from one place to another— 2001 to 2015, on average four to five times per year, is an important part of the life cycle of many species. resulting in a mean annual total of 6,250 wildebeest car- Such migrations are variously a means of avoiding cli- casses. These carcasses contribute more than 1,000 tons matic stress, escaping food and water scarcity, and sa- of biomass into the river—equivalent to roughly 10 blue tiating predators (thereby reducing individuals’ risk of whales—comprising dry mass of 107 tons carbon, being eaten). They are among the most spectacular 25 tons nitrogen, and 13 tons phosphorus. of natural phenomena, and also among the most Subalusky et al. (3) conducted a suite of detailed threatened: by building walls and dams, disrupting measurements and calculations to track the fate of the climate, and decimating wildlife populations, peo- these nutrients. By combining photographic surveys ple have steadily diminished and extinguished many of of carcasses with an energetic model for vultures, they the huge migrations known from historical records estimate that avian scavengers consume 4–7% of the (1, 2). Although tragic on purely aesthetic grounds— carbon and nitrogen, much of which is transported back nobody today knows the music of several million to land (Fig. 1C). Unscavenged soft tissues—such as American bison (Bison bison) snuffling and shuffling skin, muscle, and internal organs, which together make across the Great Plains—the extinction of great migra- up 56% of each carcass—decompose rapidly within tions also poses a profound threat to the functioning of 70 d, saturating the water with nutrients that are either ecosystems. -

Gazella Dorcas) in North East Libya
The conservation ecology of the Dorcas gazelle (Gazella dorcas) in North East Libya Walid Algadafi A thesis submitted in partial fulfilment of the requirements of the University of Wolverhampton for the degree of Doctor of Philosophy April 2019 This work and any part thereof has not previously been presented in any form to the University or to any other body whether for the purposes of assessment, publication or any other purpose (unless previously indicated). Save for any express acknowledgements, references and/or bibliographies cited in the work, I confirm that the intellectual content of the work is the result of my own efforts and of no other person. The right of Walid Algadafi to be identified as author of this work is asserted in accordance with ss.77 and 78 of the Copyright, Designs and Patents Act 1988. At this date, copyright is owned by the author. Signature: Date: 27/ 04/ 2019 I ABSTRACT The Dorcas gazelle (Gazella dorcas) is an endangered antelope in North Africa whose range is now restricted to a few small populations in arid, semi-desert conditions. To be effective, conservation efforts require fundamental information about the species, especially its abundance, distribution and genetic factors. Prior to this study, there was a paucity of such data relating to the Dorcas gazelle in Libya and the original contribution of this study is to begin to fill this gap. The aim of this study is to develop strategies for the conservation management of Dorcas gazelle in post-conflict North East Libya. In order to achieve this aim, five objectives relating to current population status, threats to the species, population genetics, conservation and strategic population management were identified. -

IUCN Red List Mediteranean Mammals.Indd
THE STATUS AND DISTRIBUTION OF MEDITERRANEAN MAMMALS Compiled by Helen J. Temple and Annabelle Cuttelod AN E AN R R E IT MED The IUCN Red List of Threatened Species™ – Regional Assessment IUCN Red list mediteranean mammals.indd 1 14/9/09 10:06:40 IUCN Red list mediteranean mammals.indd 2 17/8/09 10:50:42 THE STATUS AND DISTRIBUTION OF MEDITERRANEAN MAMMALS Compiled by Helen J. Temple and Annabelle Cuttelod The IUCN Red List of Threatened Species™ – Regional Assessment IUCN Red list mediteranean mammals.indd 1 17/8/09 10:50:42 The designation of geographical entities in this book, and the presentation of material, do not imply the expression of any opinion whatsoever on the part of IUCN or other participating organizations, concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN or other participating organizations. Published by: IUCN, Gland, Switzerland and Cambridge, UK Copyright: © 2009 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Red List logo: © 2008 Citation: Temple, H.J. and Cuttelod, A. (Compilers). 2009. The Status and Distribution of Mediterranean Mammals. Gland, Switzerland and Cambridge, UK : IUCN. vii+32pp. -

Mixed-Species Exhibits with Pigs (Suidae)
Mixed-species exhibits with Pigs (Suidae) Written by KRISZTIÁN SVÁBIK Team Leader, Toni’s Zoo, Rothenburg, Luzern, Switzerland Email: [email protected] 9th May 2021 Cover photo © Krisztián Svábik Mixed-species exhibits with Pigs (Suidae) 1 CONTENTS INTRODUCTION ........................................................................................................... 3 Use of space and enclosure furnishings ................................................................... 3 Feeding ..................................................................................................................... 3 Breeding ................................................................................................................... 4 Choice of species and individuals ............................................................................ 4 List of mixed-species exhibits involving Suids ........................................................ 5 LIST OF SPECIES COMBINATIONS – SUIDAE .......................................................... 6 Sulawesi Babirusa, Babyrousa celebensis ...............................................................7 Common Warthog, Phacochoerus africanus ......................................................... 8 Giant Forest Hog, Hylochoerus meinertzhageni ..................................................10 Bushpig, Potamochoerus larvatus ........................................................................ 11 Red River Hog, Potamochoerus porcus ............................................................... -

List of 28 Orders, 129 Families, 598 Genera and 1121 Species in Mammal Images Library 31 December 2013
What the American Society of Mammalogists has in the images library LIST OF 28 ORDERS, 129 FAMILIES, 598 GENERA AND 1121 SPECIES IN MAMMAL IMAGES LIBRARY 31 DECEMBER 2013 AFROSORICIDA (5 genera, 5 species) – golden moles and tenrecs CHRYSOCHLORIDAE - golden moles Chrysospalax villosus - Rough-haired Golden Mole TENRECIDAE - tenrecs 1. Echinops telfairi - Lesser Hedgehog Tenrec 2. Hemicentetes semispinosus – Lowland Streaked Tenrec 3. Microgale dobsoni - Dobson’s Shrew Tenrec 4. Tenrec ecaudatus – Tailless Tenrec ARTIODACTYLA (83 genera, 142 species) – paraxonic (mostly even-toed) ungulates ANTILOCAPRIDAE - pronghorns Antilocapra americana - Pronghorn BOVIDAE (46 genera) - cattle, sheep, goats, and antelopes 1. Addax nasomaculatus - Addax 2. Aepyceros melampus - Impala 3. Alcelaphus buselaphus - Hartebeest 4. Alcelaphus caama – Red Hartebeest 5. Ammotragus lervia - Barbary Sheep 6. Antidorcas marsupialis - Springbok 7. Antilope cervicapra – Blackbuck 8. Beatragus hunter – Hunter’s Hartebeest 9. Bison bison - American Bison 10. Bison bonasus - European Bison 11. Bos frontalis - Gaur 12. Bos javanicus - Banteng 13. Bos taurus -Auroch 14. Boselaphus tragocamelus - Nilgai 15. Bubalus bubalis - Water Buffalo 16. Bubalus depressicornis - Anoa 17. Bubalus quarlesi - Mountain Anoa 18. Budorcas taxicolor - Takin 19. Capra caucasica - Tur 20. Capra falconeri - Markhor 21. Capra hircus - Goat 22. Capra nubiana – Nubian Ibex 23. Capra pyrenaica – Spanish Ibex 24. Capricornis crispus – Japanese Serow 25. Cephalophus jentinki - Jentink's Duiker 26. Cephalophus natalensis – Red Duiker 1 What the American Society of Mammalogists has in the images library 27. Cephalophus niger – Black Duiker 28. Cephalophus rufilatus – Red-flanked Duiker 29. Cephalophus silvicultor - Yellow-backed Duiker 30. Cephalophus zebra - Zebra Duiker 31. Connochaetes gnou - Black Wildebeest 32. Connochaetes taurinus - Blue Wildebeest 33. Damaliscus korrigum – Topi 34. -

UC Berkeley UC Berkeley Previously Published Works
UC Berkeley UC Berkeley Previously Published Works Title Description of 3 New Species of Eimeria apicomplexa: Eimeriidae from Springbok antidorcas marsupialis in Namibia Permalink https://escholarship.org/uc/item/5125t2kr Journal Comparative Parasitology, 83(2) ISSN 1525-2647 Authors Turner, WC Penzhorn, BL Getz, WM Publication Date 2016-07-01 DOI 10.1654/4831s.1 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Comp. Parasitol. 83(2), 2016, pp. 202–211 Description of 3 New Species of Eimeria (Apicomplexa: Eimeriidae) from Springbok (Antidorcas marsupialis) in Namibia 1,6 2,3 4,5 WENDY C. TURNER, BAREND L. PENZHORN, AND WAYNE M. GETZ 1 Department of Biological Sciences, State University of New York, Albany, New York 12222, U.S.A. (e-mail: [email protected]), 2 Faculty of Veterinary Science, Department of Veterinary Tropical Diseases, University of Pretoria, Private Bag X04, Onderstepoort 0110, South Africa (e-mail: [email protected]), 3 National Zoological Gardens of South Africa, 232 Boom Street, Pretoria 0001, South Africa, 4 Department of Environmental Science, Policy and Management, University of California-Berkeley, 137 Mulford Hall, Berkeley, California 94720-3112, U.S.A. (e-mail: [email protected]), and 5 School of Mathematical Sciences, University of KwaZulu-Natal, Private Bag X54001, Durban 4000, South Africa ABSTRACT: We document morphological descriptions for 3 newly described Eimeria spp. that infect springbok (Antidorcas marsupialis). Eimeria antidorcasi n. sp. oocysts are ovoid, with average size (n 5 346) 26.2 6 18.8 (19.2–33.5 6 13.1– 26.5) mm, a length/width ratio of 1.4 (1.2–2.0), a micropyle, and a polar granule sometimes present. -

1708571114.Full.Pdf
COMMENTARY Howlargeherbivoressubsidizeaquaticfoodwebsin African savannas COMMENTARY Robert M. Pringlea,1 Mass migration—the periodic, synchronized movement in the Kenyan portion of the Mara almost every year from of large numbers of animals from one place to another— 2001 to 2015, on average four to five times per year, is an important part of the life cycle of many species. resulting in a mean annual total of 6,250 wildebeest car- Such migrations are variously a means of avoiding cli- casses. These carcasses contribute more than 1,000 tons matic stress, escaping food and water scarcity, and sa- of biomass into the river—equivalent to roughly 10 blue tiating predators (thereby reducing individuals’ risk of whales—comprising dry mass of 107 tons carbon, being eaten). They are among the most spectacular 25 tons nitrogen, and 13 tons phosphorus. of natural phenomena, and also among the most Subalusky et al. (3) conducted a suite of detailed threatened: by building walls and dams, disrupting measurements and calculations to track the fate of the climate, and decimating wildlife populations, peo- these nutrients. By combining photographic surveys ple have steadily diminished and extinguished many of of carcasses with an energetic model for vultures, they the huge migrations known from historical records estimate that avian scavengers consume 4–7% of the (1, 2). Although tragic on purely aesthetic grounds— carbon and nitrogen, much of which is transported back nobody today knows the music of several million to land (Fig. 1C). Unscavenged soft tissues—such as American bison (Bison bison) snuffling and shuffling skin, muscle, and internal organs, which together make across the Great Plains—the extinction of great migra- up 56% of each carcass—decompose rapidly within tions also poses a profound threat to the functioning of 70 d, saturating the water with nutrients that are either ecosystems. -

RED-FRONTED GAZELLE (Eudorcas Rufifrons) on CMS APPENDIX I
CMS Distribution: General CONVENTION ON UNEP/CMS/COP11/Doc.24.1.3 MIGRATORY 11 August 2014 SPECIES Original: English 11th MEETING OF THE CONFERENCE OF THE PARTIES Quito, Ecuador, 4-9 November 2014 Agenda Item 24.1.1 PROPOSAL FOR THE INCLUSION OF THE RED-FRONTED GAZELLE (Eudorcas rufifrons) ON CMS APPENDIX I Summary The Governments of Niger and Senegal have jointly submitted a proposal for the inclusion of the Red-fronted Gazelle (Eudorcas rufifrons) in CMS Appendix I for the consideration th of the 11 Meeting of the Conference of the Parties (COP11), 4-9 November 2014, Quito, Ecuador. The proposal is reproduced under this cover for a decision on its approval or rejection by the Conference of the Parties. For reasons of economy, documents are printed in a limited number, and will not be distributed at the Meeting. Delegates are requested to bring their copy to the meeting and not to request additional copies. UNEP/CMS/COP11/Doc.24.1.3: Proposal I/3 PROPOSAL FOR INCLUSION OF SPECIES ON THE APPENDICES OF THE CONVENTION ON THE CONSERVATION OF MIGRATORY SPECIES OF WILD ANIMALS A. PROPOSAL: For the Amendment of Appendix I Eudorcas rufifrons is proposed to be added to CMS Appendix I, on the basis of its decreasing population trends, throughout its range, and almost non-existent specific conservation actions taken for the species. The species is very little known, but it is thought that the majority of its dwindling populations live outside protected areas. B. PROPONENT: Niger and Senegal C. SUPPORTING STATEMENT 1. Taxon 1.1 Classis: Mammalia 1.2 Ordo: Artiodactyla 1.3 Familia: Bovidae 1.4 Genus or Species resp. -

Population Dynamics in a Guild of Four Mediterranean Ungulates: Density-Dependence, Environmental Effects and Inter-Specific Interactions
Oikos 121: 1613–1626, 2012 doi: 10.1111/j.1600-0706.2011.20085.x © 2012 The Authors. Oikos © 2012 Nordic Society Oikos Subject Editor: Jospeh Bailey. Accepted 11 October 2011 Population dynamics in a guild of four Mediterranean ungulates: density-dependence, environmental effects and inter-specific interactions S. Imperio, S. Focardi, G. Santini and A. Provenzale S. Imperio ([email protected]) and G. Santini, Dept of Evolutionary Biology, Univ. of Florence, Via Romana 17, IT-50125 Florence, Italy. – S. Focardi, Istituto Superiore per la Protezione e la Ricerca Ambientale, Via Ca’ Fornacetta 9, IT-40064 Ozzano nell’Emilia (BO), Italy. – A. Provenzale, Istituto di Scienze dell’Atmosfera e del Clima, CNR, Corso Fiume 4, IT-10133 Torino, Italy. Population fluctuations in ungulates are driven by both intrinsic and extrinsic factors. Available information, however, mainly refers to arctic, temperate and African ungulate populations, while the dynamics of Mediterranean species, exposed to a milder climate, is known to a much lesser extent. Here we studied the population dynamics of four wild ungulate spe- cies in the Castelporziano Preserve near Rome, Italy, as obtained from detailed bag counts from hunting drives during the period 1878–1986: the Italian roe deer Capreolus capreolus italicus, the Maremma wild boar Sus scrofa majori (both endemic to Italy), the native red deer Cervus elaphus, and the alien fallow deer Dama dama. We also considered the effects of the presence of another alien ungulate, the nilgai Boselaphus tragocamelus. This ungulate community experienced an accidental ‘removal experiment’ when, during World War II, red deer and nilgai were exterminated. -

List of Taxa for Which MIL Has Images
LIST OF 27 ORDERS, 163 FAMILIES, 887 GENERA, AND 2064 SPECIES IN MAMMAL IMAGES LIBRARY 31 JULY 2021 AFROSORICIDA (9 genera, 12 species) CHRYSOCHLORIDAE - golden moles 1. Amblysomus hottentotus - Hottentot Golden Mole 2. Chrysospalax villosus - Rough-haired Golden Mole 3. Eremitalpa granti - Grant’s Golden Mole TENRECIDAE - tenrecs 1. Echinops telfairi - Lesser Hedgehog Tenrec 2. Hemicentetes semispinosus - Lowland Streaked Tenrec 3. Microgale cf. longicaudata - Lesser Long-tailed Shrew Tenrec 4. Microgale cowani - Cowan’s Shrew Tenrec 5. Microgale mergulus - Web-footed Tenrec 6. Nesogale cf. talazaci - Talazac’s Shrew Tenrec 7. Nesogale dobsoni - Dobson’s Shrew Tenrec 8. Setifer setosus - Greater Hedgehog Tenrec 9. Tenrec ecaudatus - Tailless Tenrec ARTIODACTYLA (127 genera, 308 species) ANTILOCAPRIDAE - pronghorns Antilocapra americana - Pronghorn BALAENIDAE - bowheads and right whales 1. Balaena mysticetus – Bowhead Whale 2. Eubalaena australis - Southern Right Whale 3. Eubalaena glacialis – North Atlantic Right Whale 4. Eubalaena japonica - North Pacific Right Whale BALAENOPTERIDAE -rorqual whales 1. Balaenoptera acutorostrata – Common Minke Whale 2. Balaenoptera borealis - Sei Whale 3. Balaenoptera brydei – Bryde’s Whale 4. Balaenoptera musculus - Blue Whale 5. Balaenoptera physalus - Fin Whale 6. Balaenoptera ricei - Rice’s Whale 7. Eschrichtius robustus - Gray Whale 8. Megaptera novaeangliae - Humpback Whale BOVIDAE (54 genera) - cattle, sheep, goats, and antelopes 1. Addax nasomaculatus - Addax 2. Aepyceros melampus - Common Impala 3. Aepyceros petersi - Black-faced Impala 4. Alcelaphus caama - Red Hartebeest 5. Alcelaphus cokii - Kongoni (Coke’s Hartebeest) 6. Alcelaphus lelwel - Lelwel Hartebeest 7. Alcelaphus swaynei - Swayne’s Hartebeest 8. Ammelaphus australis - Southern Lesser Kudu 9. Ammelaphus imberbis - Northern Lesser Kudu 10. Ammodorcas clarkei - Dibatag 11. Ammotragus lervia - Aoudad (Barbary Sheep) 12. -
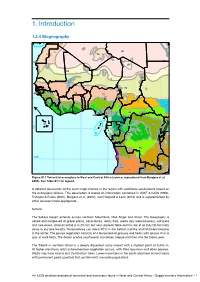
1. Introduction 86
34 85 1. Introduction 86 69 30N 60 1.2.4 Biogeography87 88 93 65 95 98 96 92 97 94 111 99 62 61 35 100 101 115 36 25 70 2 39 83 102 37 38 59 71 1 4 3 4 6 7 5 10 40 44 116 9 103 104 31 12 11 13 16 0 73 41 8 18 14 45 15 17 66 20 47 72 27 43 48 46 42 19 118 112 10S 81 74 50 21 52 82 49 32 26 56 Figure S1.1 Terrestrial ecoregions in West and Central Africa (source: reproduced from Burgess et al. 75 2004). See Table S1.1 for legend. 106 51 119 33 55 64 53 67 63 84 A2 0detailedS description of the eight major biomes in the region with additional subdivisions based on76 29 the ecoregions follows. This description is based on information contained 5in8 WWF6 8& IUCN (1994), 30 Fishpool & Evans (2001), Burgess et al. (2004), and Happold & Lock (2013) and is supplemented57 by 114 other sources where appropriate. 107 54 Terrestrial ecoregions 105 109 113 Sahara Country boundary 22 77 78 The Sahara Desert extends across northern Mauritania, Mali, Niger and Chad. The topography11 7is 28 30S 79 varied and composed of gravel plains, sand dunes, rocky flats, wadis110 (dry watercourses), salt pans 108 23 and rare oases. Annual rainfall is 0–25 mm but very unpredictable and no rain at all may fall for many 0 250 500 1,000 1,500 2,000 2,500 80 years in any one locality. -

Ungulate Taxonomy Colin Groves and Peter Grubb
Ungulate Taxonomy colin groves and peter grubb THE JOHNS HOPKINS UNIVERSITY PRESS | baltimore —-1 —0 © 2011 The Johns Hopkins University Press UNCORRECTED PROOF —+1 Do not quote for publication until verified with finished book. All rights reserved. No portion of this may be reproduced or distributed without permission. NOT FOR SALE OR DISTRIBUTION 349-47558_ch00_1P.indd iii 5/31/11 6:06 PM © 2011 The Johns Hopkins University Press All rights reserved. Published 2011 Printed in the United States of America on acid- free paper 9 8 7 6 5 4 3 2 1 The Johns Hopkins University Press 2715 North Charles Street Baltimore, Mary land 21218- 4363 w w w . p r e s s . j h u . e d u [[CIP data to come]] A cata log record for this book is available from the British Library. Special discounts are available for bulk purchases of this book. For more information, please contact Special Sales at 410- 516- 6936 or [email protected]. The Johns Hopkins University Press uses environmentally friendly book materials, including recycled text paper that is composed of at least 30 percent post- consumer waste, when ever possible. All of our book papers are acid- free, and -1— our jackets and covers are printed on paper with recycled 0— content. © 2011 The Johns Hopkins University Press +1— UNCORRECTED PROOF Do not quote for publication until verified with finished book. All rights reserved. No portion of this may be reproduced or distributed without permission. NOT FOR SALE OR DISTRIBUTION 349-47558_ch00_1P.indd iv 5/31/11 6:06 PM Contents Preface vii Theory of Ungulate Taxonomy 1 part i PERISSODACTYLA 1 Equidae 13 2 Tapiridae 18 3 Rhinocerotidae 21 part ii ARTIODACTYLA 4 Tylopoda 29 5 Suidae 33 6 Hippopotamidae 54 7 Tragulidae and Moschidae 56 8 Antilocapridae 63 9 Giraffi dae 64 10 Cervidae 71 11 Bovidae 108 References 281 Index 000 —-1 —0 © 2011 The Johns Hopkins University Press UNCORRECTED PROOF —+1 Do not quote for publication until verified with finished book.