Phase Change, Flowering and Postharvest Characteristics of Metrosideros Excelsa (Myrtaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sp09-For Web.Pub
Spring 2009 Page 1 Botanic Garden News The Botanic Garden Volume 12, No. 1 of Smith College Spring 2009 Madelaine Zadik “T he tulip is the sexiest, most capricious, the most various, subtle, powerful, and intriguing Room. Many thanks to the Museum of flower on Earth.” These are the words of Anna Art for framing them for us. Pavord, opening speaker for this year’s Spring In our display case are other tulip- Bulb Show. A mainstay of flower shows and related books lent to us by the garden displays, the tulip has come a long way Mortimer Rare Book Room. Flora’s from its humble origins in central Asia to Feast: A Masque of Flowers (1889) is Tulipa ‘Carmen Rio’ becoming a beloved spring icon. Could you opened to the tulip and hyacinth, two of Photograph by Madelaine Zadik imagine spring without tulips? forty full color lithographs in the book Horticulturist and writer extraordinaire Anna Pavord dazzled everyone with her by Walter Crane. Each page presents an talk, The Tulip: The Flower That Made Men Mad. It was more performance than allegory of a popular flower as human, lecture and demonstrated that although tulipmania might have taken over Europe in clad in flowery garments with a short the early seventeenth century, passions today still run quite strong as far as the tulip whimsical verse. Reproductions of is concerned. (For more about Anna Pavord’s visit, see page 7.) During the several of these (see page 6) were tulipmania period in Europe, fortunes rose to soaring heights and then were quickly scattered through the bulb show, to lost, perhaps similar to the Wall Street turbulence we are everyone’s delight. -

Metrosideros Polymorpha Gaudich
Metrosideros polymorpha Gaudich. JAMES A. ALLEN Paul Smiths College, Paul Smiths, NY MYRTACEAE (MYRTLE FAMILY) Metrosideros collina (J.R. and G. Forst.) A. Gray subsp. polymorpha (Gaud.) Rock. (Little and Skolmen 1989). See also the extensive list of synonyms in Wagner and others (1990) Lehua, ‘ohi’a Metrosideros is a genus of about 50 species. With the excep- hybridization and genetic polymorphism is unknown (Wagner tion of one species found in South Africa, all grow in the and others 1990). Pacific from the Philippines, through Papua New Guinea, to The heartwood is reddish brown, heavy (specific gravity New Zealand and on high volcanic islands (Wagner and oth- of about 0.70), of fine and even texture, very hard, and strong. ers 1990). Five species occur in the Hawaiian Islands (Wag- Native Hawaiians used the wood extensively for construction, ner and others 1990). Metrosideros polymorpha is native to household implements, and carvings. Principal modern uses Hawaii, where it grows on all the main islands except Niihau include flooring, marine construction, pallets, fenceposts, and and Kahoolawe. It is the most abundant and widespread fuelwood. The wood’s limitations include excessive shrinkage M native tree in Hawaii (Adee and Conrad 1990) and grows in in drying, density, and the difficulty and expense of harvesting association with numerous species in both wet and relatively in low-volume stands (Adee and Conrad 1990, Little and Skol- dry forests. men 1989). Today, M. polymorpha is perhaps most highly val- Metrosideros polymorpha is a slow-growing, evergreen ued in Hawaii for uses in watershed protection, aesthetics, and species capable of reaching 24 to 30 m in height and about 1 m habitat for native birds, including several endangered species. -

Native Plants Sixth Edition Sixth Edition AUSTRALIAN Native Plants Cultivation, Use in Landscaping and Propagation
AUSTRALIAN NATIVE PLANTS SIXTH EDITION SIXTH EDITION AUSTRALIAN NATIVE PLANTS Cultivation, Use in Landscaping and Propagation John W. Wrigley Murray Fagg Sixth Edition published in Australia in 2013 by ACKNOWLEDGEMENTS Reed New Holland an imprint of New Holland Publishers (Australia) Pty Ltd Sydney • Auckland • London • Cape Town Many people have helped us since 1977 when we began writing the first edition of Garfield House 86–88 Edgware Road London W2 2EA United Kingdom Australian Native Plants. Some of these folk have regrettably passed on, others have moved 1/66 Gibbes Street Chatswood NSW 2067 Australia to different areas. We endeavour here to acknowledge their assistance, without which the 218 Lake Road Northcote Auckland New Zealand Wembley Square First Floor Solan Road Gardens Cape Town 8001 South Africa various editions of this book would not have been as useful to so many gardeners and lovers of Australian plants. www.newhollandpublishers.com To the following people, our sincere thanks: Steve Adams, Ralph Bailey, Natalie Barnett, www.newholland.com.au Tony Bean, Lloyd Bird, John Birks, Mr and Mrs Blacklock, Don Blaxell, Jim Bourner, John Copyright © 2013 in text: John Wrigley Briggs, Colin Broadfoot, Dot Brown, the late George Brown, Ray Brown, Leslie Conway, Copyright © 2013 in map: Ian Faulkner Copyright © 2013 in photographs and illustrations: Murray Fagg Russell and Sharon Costin, Kirsten Cowley, Lyn Craven (Petraeomyrtus punicea photograph) Copyright © 2013 New Holland Publishers (Australia) Pty Ltd Richard Cummings, Bert -

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

Table of Contents Below) with Family Name Provided
1 Australian Plants Society Plant Table Profiles – Sutherland Group (updated August 2021) Below is a progressive list of all cultivated plants from members’ gardens and Joseph Banks Native Plants Reserve that have made an appearance on the Plant Table at Sutherland Group meetings. Links to websites are provided for the plants so that further research can be done. Plants are grouped in the categories of: Trees and large shrubs (woody plants generally taller than 4 m) Medium to small shrubs (woody plants from 0.1 to 4 m) Ground covers or ground-dwelling (Grasses, orchids, herbaceous and soft-wooded plants, ferns etc), as well as epiphytes (eg: Platycerium) Vines and scramblers Plants are in alphabetical order by botanic names within plants categories (see table of contents below) with family name provided. Common names are included where there is a known common name for the plant: Table of Contents Trees and Large shrubs........................................................................................................................... 2 Medium to small shrubs ...................................................................................................................... 23 Groundcovers and other ground‐dwelling plants as well as epiphytes. ............................................ 64 Vines and Scramblers ........................................................................................................................... 86 Sutherland Group http://sutherland.austplants.com.au 2 Trees and Large shrubs Acacia decurrens -

Draft Street Tree Strategy February 2018 Contents Section 1 1 Executive Summary 6 2 Introduction 8 3 Background 9 3.1 Policy - Strategic Framework 9 3.2
City of Canning A welcoming and thriving city Draft Street Tree Strategy February 2018 Contents Section 1 1 Executive Summary 6 2 Introduction 8 3 Background 9 3.1 Policy - Strategic Framework 9 3.2. Context 9 4 Alms of the Street Tree Strategy 10 5 The Benefits Of Trees 10 5.1 Environmental 10 5.2 Economic 11 5.3 Social And Physiological 11 6 Heritage Trees Within The City Of Canning 11 7 Trees As Assets 13 8 Existing Trees 13 8.1 Street Tree Audit 13 8.3 Management of Trees under Powerlines 13 8.2 Street Tree Age, Condition And Canopy Cover 14 8.4 Dominant Street Tree Species And Review Of Approved Street Tree List 17 9 Community Attitudes To Street Trees 20 9.1 Community Liaison And Community Awareness 20 10 Summary 20 11 Recommendations 20 11.1 Tree Planting 22 11.2 Biodiversity 23 11.3 Species Selection 23 11.4 Hardscape Modification 23 11.5 Auditing 23 11.6. Community Engagement 23 Tables Table 1 - Strategic Context 9 Table 2 - Dominant Street Tree Species 17 Table 3 - All Other Street Tree Species 18 DIAGRAMS Diagram 1 - Street Tree Age 14 Diagram 2 - Street Tree 14 Diagram 3 - Number of street trees pruned annually for powerline clearance 15 FIGURES Figure 1 – Street tree loss due to installation of underground services and crossovers 8 Figure 2 - Proposed and Registered Heritage Trees Hybanthus Road Tuart Tree and Woodloes Homestead, Bunya Bunya Pine 12 Figure 3 - Powerline Pruning before and after undergrounding powerlines 16 Figure 4 - Tree Tags used at the City of Adelaide 21 Section One Figure 5 - Street Tree Planting as a Traffic Engineering Design 23 4 | Draft Street Tree Strategy | Section 0ne Draft Street Tree Strategy | Section One | 5 1 Executive Summary The City of Canning has prepared this Street Tree Strategy to The Street Tree Strategy provides guidance on the selection identify planting opportunities within the City’s streetscapes. -
Show Activity
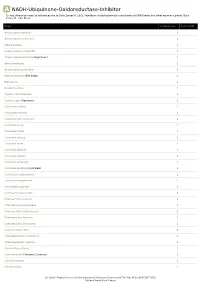
A NADH-Ubiquinone-Oxidoreductase-Inhibitor *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Adenocarpus foliolosus 1 Adenocarpus decorticans 1 Albizia procera 1 Amphicarpaea edgworthii 1 Amphicarpaea bracteata Hog Peanut 1 Apios americana 1 Argyrocytisus battandieri 1 Baptisia tinctoria Wild Indigo 1 Baptisia sp 1 Bowdichia nitida 1 Cajanus scarabaeoides 1 Cajanus cajan Pigeonpea 1 Calicotome villosa 1 Calicotome spinosa 1 Calopogonium caeruleum 1 Camptosema sp 1 Canavalia virosa 1 Canavalia sericea 1 Canavalia rosea 1 Canavalia gladiata 1 Canavalia galeata 1 Canavalia eurycarpa 1 Canavalia ensiformis Jack Bean 1 Centrosema schiedianum 1 Centrosema sagittatum 1 Centrosema plumieri 1 Centrosema pascuorum 1 Chamaecytisus supinus 1 Chamaecytisus smyrnaeus 1 Chamaecytisus ratisbonensis 1 Chamaecytisus hirsutus 1 Chamaecytisus eriocarpus 1 Chamaecytisus albus 1 Chamaespartium tridentatum 1 Chamaespartium sagittale 1 Chronanthus biflorus 1 Cicer arietinum Chickpea; Garbanzo 1 Clitoria ternatea 1 Clitoria falcata 1 Dr. Duke's Phytochemical and Ethnobotanical Databases Downloaded Thu Sep 30 21:42:43 EDT 2021 National Agricultural Library Plant # Chemicals Total PPM Cologana broussonetii 1 Crotalaria juncea Sunhemp 1 Cytisus tribracteolatus 1 Cytisus striatus 1 Cytisus scoparius Scotch Broom 1 Cytisus multiflorus 1 Cytisus ingramii 1 Cytisus ardoini 1 Desmodium gangeticum 1 Dioclea glycinoides -

Clematis Clematis Are the Noblest and Most Colorful of Climbing Vines
Jilacktborne SUPER HARDY Clematis Clematis are the noblest and most colorful of climbing vines. Fortunately, they are also one of the hardiest, most disease free and therefore easiest of culture. As the result of our many years of research and development involving these glorious vines, we now make available to the American gardening public: * Heavy TWO YEAR plants (the absolute optimum size for successful plant RED CARDINAL ing in your garden). * Own rooted plants - NOT GRAFTED - therefore not susceptible to com mon Clematis wilt. * Heavily rooted, BLOOMING SIZE plants, actually growing in a rich 100% organic medium, - all in an especially designed container. * Simply remove container, plant, and - "JUMP BACK"!! For within a few days your Blackthorne Clematis will be growing like the proverbial "weed", and getting ready to flower! * Rare and distinctive species and varieties not readily available commer cially - if at all! * Plants Northern grown to our rigid specifications by one of the world's premier Clematis growers and plantsmen, Arthur H. Steffen, Inc. * The very ultimate in simplified, pictorial cultural instructions AVAILABLE NOWHERE ELSE, Free with order. - OLD GLORY CLEMATIS COLLECTION - RED RED CARDINAL - New from France comes this, the most spec tacular red Clematis ever developed. It is a blazing mass of glory from May on. Each of the large, velvety, rich crimson red blooms is lit up by a sun-like mass of bright golden stamens, in the very heart of the flower! Red Cardinal's rich brilliance de- fies description! $6.95 each - 3 for $17.95 POSTPA ID WHITE MME LE COULTRE - Another great new one from France, and the finest white hybrid Clematis ever developed. -

Genera in Myrtaceae Family
Genera in Myrtaceae Family Genera in Myrtaceae Ref: http://data.kew.org/vpfg1992/vascplnt.html R. K. Brummitt 1992. Vascular Plant Families and Genera, Royal Botanic Gardens, Kew REF: Australian – APC http://www.anbg.gov.au/chah/apc/index.html & APNI http://www.anbg.gov.au/cgi-bin/apni Some of these genera are not native but naturalised Tasmanian taxa can be found at the Census: http://tmag.tas.gov.au/index.aspx?base=1273 Future reference: http://tmag.tas.gov.au/floratasmania [Myrtaceae is being edited at mo] Acca O.Berg Euryomyrtus Schaur Osbornia F.Muell. Accara Landrum Feijoa O.Berg Paragonis J.R.Wheeler & N.G.Marchant Acmena DC. [= Syzigium] Gomidesia O.Berg Paramyrciaria Kausel Acmenosperma Kausel [= Syzigium] Gossia N.Snow & Guymer Pericalymma (Endl.) Endl. Actinodium Schauer Heteropyxis Harv. Petraeomyrtus Craven Agonis (DC.) Sweet Hexachlamys O.Berg Phymatocarpus F.Muell. Allosyncarpia S.T.Blake Homalocalyx F.Muell. Pileanthus Labill. Amomyrtella Kausel Homalospermum Schauer Pilidiostigma Burret Amomyrtus (Burret) D.Legrand & Kausel [=Leptospermum] Piliocalyx Brongn. & Gris Angasomyrtus Trudgen & Keighery Homoranthus A.Cunn. ex Schauer Pimenta Lindl. Angophora Cav. Hottea Urb. Pleurocalyptus Brongn. & Gris Archirhodomyrtus (Nied.) Burret Hypocalymma (Endl.) Endl. Plinia L. Arillastrum Pancher ex Baill. Kania Schltr. Pseudanamomis Kausel Astartea DC. Kardomia Peter G. Wilson Psidium L. [naturalised] Asteromyrtus Schauer Kjellbergiodendron Burret Psiloxylon Thouars ex Tul. Austromyrtus (Nied.) Burret Kunzea Rchb. Purpureostemon Gugerli Babingtonia Lindl. Lamarchea Gaudich. Regelia Schauer Backhousia Hook. & Harv. Legrandia Kausel Rhodamnia Jack Baeckea L. Lenwebia N.Snow & ZGuymer Rhodomyrtus (DC.) Rchb. Balaustion Hook. Leptospermum J.R.Forst. & G.Forst. Rinzia Schauer Barongia Peter G.Wilson & B.Hyland Lindsayomyrtus B.Hyland & Steenis Ristantia Peter G.Wilson & J.T.Waterh. -

RCM028 Hutt Lagoon Condition Report
Resource Condition Report for a Significant Western Australian Wetland Hutt Lagoon 2009 Figure 1 – A view across the water body at Hutt Lagoon, coloured pink by alga. This report was prepared by: Anna Nowicki, Technical Officer, Department of Environment and Conservation, PO Box 51, Wanneroo 6946 Adrian Pinder, Senior Research Scientist, Department of Environment and Conservation, PO Box 51, Wanneroo 6946 Stephen Kern, Botanist, Department of Environment and Conservation, Locked Bag 104 Bentley Delivery Centre 6983 Glen Daniel, Environmental Officer, Department of Environment and Conservation, Locked Bag 104 Bentley Delivery Centre 6983 Invertebrate sorting and identification by: Nadine Guthrie, Research Scientist, Department of Environment and Conservation, PO Box 51, Wanneroo 6946 Ross Gordon, Project Officer, Department of Environment and Conservation, PO Box 51, Wanneroo 6946 Prepared for: Inland Aquatic Integrity Resource Condition Monitoring Project, Strategic Reserve Fund, Department of Environment and Conservation August 2009 Suggested Citation: Department of Environment and Conservation (DEC) (2009). Resource Condition Report for a Significant Western Australian Wetland: Hutt Lagoon. Department of Environment and Conservation, Perth, Western Australia. Contents 1. Introduction.........................................................................................................................1 1.1. Site Code ...............................................................................................................1 1.2. -

The Following Tree Seedlings Are Available to Order from the State of Hawaii Division of Forestry and Wildlife, State Tree Nursery
The following tree seedlings are available to order from the State of Hawaii Division of Forestry and Wildlife, State Tree Nursery: Scientific Name: Common Name: Dibble/ Pot size: Acacia koa……………………… Koa……………………………….. Small Acacia koaia……………………... Koai’a……………………………. Small Araucaria columnaris…………….. Norfolk-island Pine……………… Small Cryptomeria japonica……………. Sugi Pine………………………… Small Cupressus lusitanica……………... Mexican Cypress………………… Small Cupressus macrocarpa…………… Monterey Cypress……………….. Small Cupressus simpervirens………….. Italian Cypress…………………… Medium Eucalyptus deglupta……………… Rainbow Bark……………………. Small Eucalyptus robusta……………….. Swamp Mahogany……………….. Small Metrosideros polymorpha……….. Ohia……………………………… Medium or 3” pot Pinus elliotii……………………… Slash Pine………………………... Small Pinus radiata……………………... Monterey Pine…………………… Small Podocarpus sp……………………. Podocarpus………………………. 3” pot Santalum sp……………………… Sandalwood……………………… Medium or 3” pot Tristania conferta………………… Brush Box………………………... Small Acacia koa (Koa): This large hardwood tree is endemic to the Hawaiian Islands. The tree has exceeded 100 ft in height with basal diameter far beyond 50 inches in old growth stands. The wood is prized for furniture and canoe works. This legume has pods with black seeds for reproduction. The wood has similar properties to that of black walnut. The yellow flowers are borne in dense round heads about 2@ in diameter. Tree growth is best above 800 ft; seems to grow best in the ‘Koa belt’ which is situated at an elevation range between 3,500 - 6,000 ft. It is often found in areas where there is fog in the late afternoons. It should be planted in well- drained fertile soils. Grazing animals relish the Koa foliage, so young seedlings should be protected Acacia koaia (Koaia): Related to the Koa, Koaia is native to Hawaii. The leaves and flowers are much the same as Koa. -

Curriculum Vitae Fulbright Postdoctoral Fellow Soil and Fungal
1 LOUISE M. EGERTON-WARBURTON Curriculum Vitae Chicago Botanic Garden Phone: 847.835.6915 1000 Lake Cook Rd Fax: 847.835.5484 Glencoe IL 60022 [email protected] EDUCATION: Fulbright Postdoctoral Fellow University of California, 1994- Soil and fungal ecology Riverside 1997 Ph.D., Environmental Biology Curtin University of 1994 Dissertation: Soil-plant relationships of Eucalyptus species Technology, Australia in acidic coal mining soils. Adviser: Byron B. Lamont B.S., Biology (Highest Honors) Curtin University of 1989 Minor: Statistics Technology, Australia B.S., Nursing Western Australian School 1984 Clinical specialty: Operating Theater of Nursing APPOINTMENTS: Director and Coordinator, Research Experiences for Chicago Botanic Garden 2004- Undergraduates (REU) site in Plant Conservation and 2010 Biology Adjunct Professor of Biology Northwestern University 2003- present Conservation Scientist, Chicago Botanic Garden 2001- Soil and Microbial Ecology, and present Manager, Soil Sciences Program Assistant Researcher, University of California, 1999- Soil Microbial Ecology Riverside 2001 Post-doctoral Research Fellow, University of Melbourne, 1998- Cell & Molecular Biology, Nanotechnology Australia 1999 TEACHING APPOINTMENTS: Instructor, §Field and Lab Methods in Conservation Northwestern University 2009- Biology (PBC499) present Instructor, §Soils and Environment (PBC448), Northwestern University 2008- Fall quarter present Guest lecturer, Introductory Mycology University of Wisconsin, 2008 Winter quarter Madison 2 Guest lecturer,