A Role for Preprophase Bands of Microtubules in Maturation of New
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phragmoplast Microtubule Dynamics – a Game of Zones Andrei Smertenko1,2,‡, Seanna L
© 2018. Published by The Company of Biologists Ltd | Journal of Cell Science (2018) 131, jcs203331. doi:10.1242/jcs.203331 REVIEW SPECIAL ISSUE: PLANT CELL BIOLOGY Phragmoplast microtubule dynamics – a game of zones Andrei Smertenko1,2,‡, Seanna L. Hewitt2,3, Caitlin N. Jacques2,4, Rafal Kacprzyk1, Yan Liu2,5, Matthew J. Marcec2,6, Lindani Moyo2,6, Aaron Ogden1,2, Hui Min Oung1,2, Sharol Schmidt1,2 and Erika A. Serrano-Romero2,5 ABSTRACT during anaphase from the remnants of the central spindle (Segui- Plant morphogenesis relies on the accurate positioning of the partition Simarro et al., 2004). It consists of microtubules, actin, membrane (cell plate) between dividing cells during cytokinesis. The cell plate is compartments and proteins that associate with or regulate the above synthetized by a specialized structure called the phragmoplast, which (Boruc and Van Damme, 2015; Lipka et al., 2015). The microtubule consists of microtubules, actin filaments, membrane compartments component of the phragmoplast consists of two aligned arrays that and associated proteins. The phragmoplast forms between daughter flank the so-called phragmoplast midzone, where cell plate assembly nuclei during the transition from anaphase to telophase. As cells are takes place (Fig. 1). The initial phragmoplast has a disk shape with a commonly larger than the originally formed phragmoplast, the diameter that approximately equals that of the daughter nuclei construction of the cell plate requires phragmoplast expansion. (Fig. 1); however, the parental cell is generally wider. For example, This expansion depends on microtubule polymerization at the the length of a cambium cell exceeds the diameter of the disk-shaped phragmoplast forefront (leading zone) and loss at the back (lagging phragmoplast during late anaphase by up to 100 fold (Larson, 1994). -

Mitosis Vs. Meiosis
Mitosis vs. Meiosis In order for organisms to continue growing and/or replace cells that are dead or beyond repair, cells must replicate, or make identical copies of themselves. In order to do this and maintain the proper number of chromosomes, the cells of eukaryotes must undergo mitosis to divide up their DNA. The dividing of the DNA ensures that both the “old” cell (parent cell) and the “new” cells (daughter cells) have the same genetic makeup and both will be diploid, or containing the same number of chromosomes as the parent cell. For reproduction of an organism to occur, the original parent cell will undergo Meiosis to create 4 new daughter cells with a slightly different genetic makeup in order to ensure genetic diversity when fertilization occurs. The four daughter cells will be haploid, or containing half the number of chromosomes as the parent cell. The difference between the two processes is that mitosis occurs in non-reproductive cells, or somatic cells, and meiosis occurs in the cells that participate in sexual reproduction, or germ cells. The Somatic Cell Cycle (Mitosis) The somatic cell cycle consists of 3 phases: interphase, m phase, and cytokinesis. 1. Interphase: Interphase is considered the non-dividing phase of the cell cycle. It is not a part of the actual process of mitosis, but it readies the cell for mitosis. It is made up of 3 sub-phases: • G1 Phase: In G1, the cell is growing. In most organisms, the majority of the cell’s life span is spent in G1. • S Phase: In each human somatic cell, there are 23 pairs of chromosomes; one chromosome comes from the mother and one comes from the father. -

The Revised Classification of Eukaryotes
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/231610049 The Revised Classification of Eukaryotes Article in Journal of Eukaryotic Microbiology · September 2012 DOI: 10.1111/j.1550-7408.2012.00644.x · Source: PubMed CITATIONS READS 961 2,825 25 authors, including: Sina M Adl Alastair Simpson University of Saskatchewan Dalhousie University 118 PUBLICATIONS 8,522 CITATIONS 264 PUBLICATIONS 10,739 CITATIONS SEE PROFILE SEE PROFILE Christopher E Lane David Bass University of Rhode Island Natural History Museum, London 82 PUBLICATIONS 6,233 CITATIONS 464 PUBLICATIONS 7,765 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Biodiversity and ecology of soil taste amoeba View project Predator control of diversity View project All content following this page was uploaded by Smirnov Alexey on 25 October 2017. The user has requested enhancement of the downloaded file. The Journal of Published by the International Society of Eukaryotic Microbiology Protistologists J. Eukaryot. Microbiol., 59(5), 2012 pp. 429–493 © 2012 The Author(s) Journal of Eukaryotic Microbiology © 2012 International Society of Protistologists DOI: 10.1111/j.1550-7408.2012.00644.x The Revised Classification of Eukaryotes SINA M. ADL,a,b ALASTAIR G. B. SIMPSON,b CHRISTOPHER E. LANE,c JULIUS LUKESˇ,d DAVID BASS,e SAMUEL S. BOWSER,f MATTHEW W. BROWN,g FABIEN BURKI,h MICAH DUNTHORN,i VLADIMIR HAMPL,j AARON HEISS,b MONA HOPPENRATH,k ENRIQUE LARA,l LINE LE GALL,m DENIS H. LYNN,n,1 HILARY MCMANUS,o EDWARD A. D. -

Molecular Biology and Applied Genetics
MOLECULAR BIOLOGY AND APPLIED GENETICS FOR Medical Laboratory Technology Students Upgraded Lecture Note Series Mohammed Awole Adem Jimma University MOLECULAR BIOLOGY AND APPLIED GENETICS For Medical Laboratory Technician Students Lecture Note Series Mohammed Awole Adem Upgraded - 2006 In collaboration with The Carter Center (EPHTI) and The Federal Democratic Republic of Ethiopia Ministry of Education and Ministry of Health Jimma University PREFACE The problem faced today in the learning and teaching of Applied Genetics and Molecular Biology for laboratory technologists in universities, colleges andhealth institutions primarily from the unavailability of textbooks that focus on the needs of Ethiopian students. This lecture note has been prepared with the primary aim of alleviating the problems encountered in the teaching of Medical Applied Genetics and Molecular Biology course and in minimizing discrepancies prevailing among the different teaching and training health institutions. It can also be used in teaching any introductory course on medical Applied Genetics and Molecular Biology and as a reference material. This lecture note is specifically designed for medical laboratory technologists, and includes only those areas of molecular cell biology and Applied Genetics relevant to degree-level understanding of modern laboratory technology. Since genetics is prerequisite course to molecular biology, the lecture note starts with Genetics i followed by Molecular Biology. It provides students with molecular background to enable them to understand and critically analyze recent advances in laboratory sciences. Finally, it contains a glossary, which summarizes important terminologies used in the text. Each chapter begins by specific learning objectives and at the end of each chapter review questions are also included. -

The Cell Cycle & Mitosis
The Cell Cycle & Mitosis Cell Growth The Cell Cycle is G1 phase ___________________________________ _______________________________ During the Cell Cycle, a cell ___________________________________ ___________________________________ Anaphase Cell Division ___________________________________ Mitosis M phase M ___________________________________ S phase replication DNA Interphase Interphase is ___________________________ ___________________________________ G2 phase Interphase is divided into three phases: ___, ___, & ___ G1 Phase S Phase G2 Phase The G1 phase is a period of The S phase replicates During the G2 phase, many of activity in which cells _______ ________________and the organelles and molecules ____________________ synthesizes _______ molecules. required for ____________ __________ Cells will When DNA replication is ___________________ _______________ and completed, _____________ When G2 is completed, the cell is synthesize new ___________ ____________________ ready to enter the ____________________ ____________________ ____________________ ____________________ ____________________ Mitosis are divided into four phases: _____________, ______________, _____________, & _____________ Below are cells in two different phases of the cell cycle, fill in the blanks using the word bank: Chromatin Nuclear Envelope Chromosome Sister Chromatids Nucleolus Spinder Fiber Centrosome Centrioles 5.._________ 1.__________ v 6..__________ 2.__________ 7.__________ 3.__________ 8..__________ 4.__________ v The Cell Cycle & Mitosis Microscope Lab: -

Functions of the Arabidopsis Kinesin Superfamily of Microtubule-Based Motor Proteins Chuanmei Zhu
Washington University in St. Louis Washington University Open Scholarship Biology Faculty Publications & Presentations Biology 10-2012 Functions of the Arabidopsis kinesin superfamily of microtubule-based motor proteins Chuanmei Zhu Ram Dixit Washington University in St Louis, [email protected] Follow this and additional works at: https://openscholarship.wustl.edu/bio_facpubs Part of the Biochemistry Commons, Biology Commons, and the Plant Biology Commons Recommended Citation Zhu, Chuanmei and Dixit, Ram, "Functions of the Arabidopsis kinesin superfamily of microtubule-based motor proteins" (2012). Biology Faculty Publications & Presentations. 79. https://openscholarship.wustl.edu/bio_facpubs/79 This Article is brought to you for free and open access by the Biology at Washington University Open Scholarship. It has been accepted for inclusion in Biology Faculty Publications & Presentations by an authorized administrator of Washington University Open Scholarship. For more information, please contact [email protected]. Functions of the Arabidopsis kinesin superfamily of microtubule-based motor proteins Chuanmei Zhu and Ram Dixit Biology Department, Washington University, St. Louis, MO 63130 Corresponding author Ram Dixit 1 Brookings Drive, CB 1137 St. Louis, MO 63130. Phone: (314) 935-8823 Fax: (314) 935-4432 Email: [email protected] Keywords Plant, cortical microtubule, preprophase band, spindle, phragmoplast ABSTRACT Plants possess a large number of microtubule-based kinesin motor proteins. While the Kinesin-2, 3, 9 and 11 families are absent from land plants, the Kinesin-7 and 14 families are greatly expanded. In addition, some kinesins are specifically present only in land plants. The distinctive inventory of plant kinesins suggests that kinesins have evolved to perform specialized functions in plants. -

Cell Life Cycle and Reproduction the Cell Cycle (Cell-Division Cycle), Is a Series of Events That Take Place in a Cell Leading to Its Division and Duplication
Cell Life Cycle and Reproduction The cell cycle (cell-division cycle), is a series of events that take place in a cell leading to its division and duplication. The main phases of the cell cycle are interphase, nuclear division, and cytokinesis. Cell division produces two daughter cells. In cells without a nucleus (prokaryotic), the cell cycle occurs via binary fission. Interphase Gap1(G1)- Cells increase in size. The G1checkpointcontrol mechanism ensures that everything is ready for DNA synthesis. Synthesis(S)- DNA replication occurs during this phase. DNA Replication The process in which DNA makes a duplicate copy of itself. Semiconservative Replication The process in which the DNA molecule uncoils and separates into two strands. Each original strand becomes a template on which a new strand is constructed, resulting in two DNA molecules identical to the original DNA molecule. Gap 2(G2)- The cell continues to grow. The G2checkpointcontrol mechanism ensures that everything is ready to enter the M (mitosis) phase and divide. Mitotic(M) refers to the division of the nucleus. Cell growth stops at this stage and cellular energy is focused on the orderly division into daughter cells. A checkpoint in the middle of mitosis (Metaphase Checkpoint) ensures that the cell is ready to complete cell division. The final event is cytokinesis, in which the cytoplasm divides and the single parent cell splits into two daughter cells. Reproduction Cellular reproduction is a process by which cells duplicate their contents and then divide to yield multiple cells with similar, if not duplicate, contents. Mitosis Mitosis- nuclear division resulting in the production of two somatic cells having the same genetic complement (genetically identical) as the original cell. -

Rangap1 Is a Continuous Marker of the Arabidopsis Cell Division Plane
RanGAP1 is a continuous marker of the Arabidopsis cell division plane Xianfeng Morgan Xua,1, Qiao Zhaoa,2, Thushani Rodrigo-Peirisa, Jelena Brkljacica, Chao Sylvia Hea,1, Sabine Mu¨ llerb, and Iris Meiera,3 aPlant Biotechnology Center and Department of Plant Cellular and Molecular Biology, The Ohio State University, Columbus, OH 43210; and bSchool of Biological Sciences, University of Auckland, Auckland 1142, New Zealand Edited by Maarten J. Chrispeels, University of California at San Diego, La Jolla, CA, and approved October 8, 2008 (received for review June 30, 2008) In higher plants, the plane of cell division is faithfully predicted by were found to interact with TANGLED and a pok1 pok2 double the preprophase band (PPB). The PPB, a cortical ring of microtu- mutant resembles the maize tan mutant in terms of misoriented bules and F-actin, disassembles upon nuclear-envelope break- division planes (9). Although the data suggest a role for kinesins down. During cytokinesis, the expanding cell plate fuses with the and the pioneer protein TANGLED in division-plane definition, plasma membrane at the cortical division site, the site of the former the molecular mechanism of the process remains unknown. PPB. The nature of the ‘‘molecular memory’’ that is left behind by Ran is a small GTPase that in vertebrates controls multiple the PPB and is proposed to guide the cell plate to the cortical cellular processes including nucleocytoplasmic transport, spindle division site is unknown. RanGAP is the GTPase activating protein assembly, nuclear envelope reassembly, centrosome duplication, of the small GTPase Ran, which provides spatial information for and cell-cycle control (ref. -
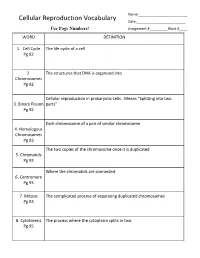
Cellular Reproduction Vocabulary Date:______Use Page Numbers! Assignment #______Block #____ WORD DEFINITION
Name:_________________________ Cellular Reproduction Vocabulary Date:_________________________ Use Page Numbers! Assignment #_________ Block #____ WORD DEFINITION 1. Cell Cycle The life cycle of a cell Pg 92 2. The structures that DNA is organized into Chromosomes Pg 92 Cellular reproduction in prokaryotic cells. Means “Splitting into two 3. Binary Fission parts” Pg 92 Each chromosome of a pair of similar chromosome 4. Homologous Chromosomes Pg 93 The two copies of the chromosome once it is duplicated 5. Chromatids Pg 93 Where the chromatids are connected 6. Centromere Pg 93 7. Mitosis The complicated process of separating duplicated chromosomes Pg 93 8. Cytokinesis The process where the cytoplasm splits in two Pg 95 WORD DEFINITION 9. Budding A type of asexual reproduction, where a piece of the parents body Pg 612 develops into an independent organism 10. A type of asexual reproduction, where the organism breaks into two or Regeneration more parts, each growing into a new organism that is genetically identical (fragmentation) to the parent Pg 612 The production of offspring by combining the genetic material of more 11. Sexual than one parent reproduction Pg 613 A single parent has offspring that are identical to itself 12. Asexual reproduction Pg 613 The male sex cell 13. Sperm Pg 613 The female sex cell 14. Egg Pg 613 The new type of cell that is made when an egg’s nucleus fuses with a 15. Zygote sperm’s nucleus Pg 613 16. Spores Small reproductive cells that are protected by a thick cell wall Pg 256 WORD DEFINITION 17. Sex Cells Specialized cells that combine to form a zygote, they have half the normal Pg 114 number of chromosomes, one of each pair. -

Cell Division for Growth of Eukaryotic Organisms and Replacement of Some Eukaryotic Cells
Mitosis CELL DIVISION FOR GROWTH OF EUKARYOTIC ORGANISMS AND REPLACEMENT OF SOME EUKARYOTIC CELLS T H I S WORK IS LICENSED UNDER A CREATIVE COMMONS ATTRIBUTION - NONCOMMERCIAL - SHAREALIKE 4 . 0 INTERNATIONAL LICENSE . History of Understanding Cancer Rudolf Virchow (1821-1902) – First to recognize leukemia in mid-1800s, believing that diseased tissue was caused by a breakdown within the cell and not from an invasion of foreign organisms. Louis Pasteur (1822-1895) – Proved Virchow to be correct in late 1800s. Virchow’s understanding that cancer cells start out normal and then become abnormal is still used today. If cancer is the study of abnormal cell division, let’s look at normal cell division. Types of Normal Cell Division There are two types of normal cell division – mitosis and meiosis. Mitosis is cell division which begins in the fertilized egg (or zygote) stage and continues during the life of the organism in one way or another. Each diploid (2n) daughter cell is genetically identical to the diploid (2n) parent cell. Meiosis is cell division in the ovaries of the female and testes of the male and involves the formation of egg and sperm cells, respectively. Each diploid (2n) parent cell produces haploid (n) daughter cells. Meiosis will be discussed more fully in Chapter 5 of the Oncofertility Curriculum. Walther Flemming (1843 – 1905) • Described the process of cell division in 1882 and coined the word ‘mitosis’ • Also responsible for the word “chromosome’ which he first referred to as stained strands • Co-worker Eduard Strasburger -

Cell Death by Mitotic Catastrophe: a Molecular Definition
Oncogene (2004) 23, 2825–2837 & 2004 Nature Publishing Group All rights reserved 0950-9232/04 $25.00 www.nature.com/onc Cell death by mitotic catastrophe: a molecular definition Maria Castedo1, Jean-Luc Perfettini1, Thomas Roumier1, Karine Andreau1, Rene Medema2 and Guido Kroemer*,1 1CNRS-UMR 8125, Institut Gustave Roussy, Pavillon de Recherche 1, 39 rue Camille-Desmoulins, Villejuif F-94805, France; 2Division of Molecular Biology H8, Netherlands Cancer Institute, Plesmanlaan 121, Amsterdam 1066 CX, The Netherlands The current literature is devoid of a clearcut definition of mutant strains (Molz et al., 1989; Ayscough et al., mitotic catastrophe, a type of cell death that occurs during 1992). Accordingly, some authors view ‘mitotic mitosis. Here, we propose that mitotic catastrophe results catastrophe’ of mammalian cells as the failure to from a combination of deficient cell-cycle checkpoints (in undergo complete mitosis (which would be more particular the DNA structure checkpoints and the spindle accurately called ‘mitotic failure’) after DNA damage assembly checkpoint) and cellular damage. Failure to (coupled to defective checkpoints), a situation that arrest the cell cycle before or at mitosis triggers an would lead to tetraploidy (after a single cell cycle) attempt of aberrant chromosome segregation, which (Andreassen et al., 2001a; Margottin-Goguet et al., culminates in the activation of the apoptotic default 2003) or endopolyploidy (after several cell cycles) with pathway and cellular demise. Cell death occurring during extensive DNA damage and repair, perhaps followed by the metaphase/anaphase transition is characterized by the the selection of apoptosis-resistance cells that will ulti- activation of caspase-2 (which can be activated in response mately survive after endoreduplication (Ivanov et al., to DNA damage) and/or mitochondrial membrane per- 2003). -

The Spindle Checkpoint
Cell Science at a Glance 4139 The spindle checkpoint mechanism that delays anaphase onset highlight current understanding of how until all chromosomes are correctly the spindle checkpoint is activated, how it Karen M. May and Kevin G. attached in a bipolar fashion to the delays anaphase onset, and how it is Hardwick mitotic spindle. silenced. Wellcome Trust Centre for Cell Biology, University of Edinburgh, EH9 3JR, UK The core spindle checkpoint proteins are (e-mail: [email protected]) Mad1, Mad2, BubR1 (Mad3 in yeast), Activation of the checkpoint Journal of Cell Science 119, 4139-4142 Bub1, Bub3 and Mps1. The Mad and Bub During mitosis spindle microtubules Published by The Company of Biologists 2006 proteins were first identified in budding bind to complex protein structures called doi:10.1242/jcs.03165 yeast by genetic screens for mutants that kinetochores, which assemble on the failed to arrest in mitosis when the spindle centromere of each chromosome. The Every mitosis, replicated chromosomes was destroyed (Taylor et al., 2004). These Mad and Bub proteins localise to the must be accurately segregated into each proteins are conserved in all eukaryotes. outer kinetochore early in mitosis, before daughter cell. Pairs of sister chromatids Several other checkpoint components, proper attachments are established, and attach to the bipolar mitotic spindle such as Rod, Zw10 and CENP-E, have accumulate on unattached kinetochores. during prometaphase, they are aligned at since been identified in higher eukaryotes When spindle microtubules make metaphase, then sisters separate and but have no yeast orthologues (Karess, contact with the outer kinetochore are pulled to opposite poles during 2005; Mao et al., 2003).