Identification of an HMGB3 Frameshift Mutation in a Family with an X-Linked Colobomatous Microphthalmia Syndrome Using Whole-Genome and X-Exome Sequencing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Dual Regulation of Planar Polarization by Secreted Wnts and Vangl2 in the Developing Mouse Cochlea Elvis Huarcaya Najarro1, Jennifer Huang1, Adrian Jacobo2, Lee A
© 2020. Published by The Company of Biologists Ltd | Development (2020) 147, dev191981. doi:10.1242/dev.191981 RESEARCH ARTICLE Dual regulation of planar polarization by secreted Wnts and Vangl2 in the developing mouse cochlea Elvis Huarcaya Najarro1, Jennifer Huang1, Adrian Jacobo2, Lee A. Quiruz1, Nicolas Grillet1 and Alan G. Cheng1,* ABSTRACT on the other. Flamingo (Fmi/Celsr1, Fmi/Celsr2 and Fmi/Celsr3) is Planar cell polarity (PCP) proteins localize asymmetrically to instruct present on both poles of the cell. Defective PCP signaling cell polarity within the tissue plane, with defects leading to deformities represented by a lack of polarized PCP components leads to of the limbs, neural tube and inner ear. Wnt proteins are evolutionarily congenital heart and tracheal abnormalities, skeletal dysplasia, conserved polarity cues, yet Wnt mutants display variable PCP neural tube defects as well as cochlear deformities (Butler and defects; thus, how Wnts regulate PCP remains unresolved. Here, we Wallingford, 2017; White et al., 2018). Despite their crucial roles, have used the developing cochlea as a model system to show that our understanding of upstream signals orchestrating PCP signaling secreted Wnts regulate PCP through polarizing a specific subset of is rather limited. PCP proteins. Conditional deletion of Wntless or porcupine, both of Wnt proteins have been implicated as upstream polarity cues for which are essential for secretion of Wnts, caused misrotated sensory PCP signaling. For example, limb morphogenesis in mice requires a cells and shortened cochlea – both hallmarks of PCP defects. gradient of Wnt5a, which has been reported to act as an instructive Wntless-deficient cochleae lacked the polarized PCP components cue to establish PCP (Gao et al., 2018, 2011). -

HMGB1 in Health and Disease R
Donald and Barbara Zucker School of Medicine Journal Articles Academic Works 2014 HMGB1 in health and disease R. Kang R. C. Chen Q. H. Zhang W. Hou S. Wu See next page for additional authors Follow this and additional works at: https://academicworks.medicine.hofstra.edu/articles Part of the Emergency Medicine Commons Recommended Citation Kang R, Chen R, Zhang Q, Hou W, Wu S, Fan X, Yan Z, Sun X, Wang H, Tang D, . HMGB1 in health and disease. 2014 Jan 01; 40():Article 533 [ p.]. Available from: https://academicworks.medicine.hofstra.edu/articles/533. Free full text article. This Article is brought to you for free and open access by Donald and Barbara Zucker School of Medicine Academic Works. It has been accepted for inclusion in Journal Articles by an authorized administrator of Donald and Barbara Zucker School of Medicine Academic Works. Authors R. Kang, R. C. Chen, Q. H. Zhang, W. Hou, S. Wu, X. G. Fan, Z. W. Yan, X. F. Sun, H. C. Wang, D. L. Tang, and +8 additional authors This article is available at Donald and Barbara Zucker School of Medicine Academic Works: https://academicworks.medicine.hofstra.edu/articles/533 NIH Public Access Author Manuscript Mol Aspects Med. Author manuscript; available in PMC 2015 December 01. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Mol Aspects Med. 2014 December ; 0: 1–116. doi:10.1016/j.mam.2014.05.001. HMGB1 in Health and Disease Rui Kang1,*, Ruochan Chen1, Qiuhong Zhang1, Wen Hou1, Sha Wu1, Lizhi Cao2, Jin Huang3, Yan Yu2, Xue-gong Fan4, Zhengwen Yan1,5, Xiaofang Sun6, Haichao Wang7, Qingde Wang1, Allan Tsung1, Timothy R. -

Supplementary Data
SUPPLEMENTARY DATA A cyclin D1-dependent transcriptional program predicts clinical outcome in mantle cell lymphoma Santiago Demajo et al. 1 SUPPLEMENTARY DATA INDEX Supplementary Methods p. 3 Supplementary References p. 8 Supplementary Tables (S1 to S5) p. 9 Supplementary Figures (S1 to S15) p. 17 2 SUPPLEMENTARY METHODS Western blot, immunoprecipitation, and qRT-PCR Western blot (WB) analysis was performed as previously described (1), using cyclin D1 (Santa Cruz Biotechnology, sc-753, RRID:AB_2070433) and tubulin (Sigma-Aldrich, T5168, RRID:AB_477579) antibodies. Co-immunoprecipitation assays were performed as described before (2), using cyclin D1 antibody (Santa Cruz Biotechnology, sc-8396, RRID:AB_627344) or control IgG (Santa Cruz Biotechnology, sc-2025, RRID:AB_737182) followed by protein G- magnetic beads (Invitrogen) incubation and elution with Glycine 100mM pH=2.5. Co-IP experiments were performed within five weeks after cell thawing. Cyclin D1 (Santa Cruz Biotechnology, sc-753), E2F4 (Bethyl, A302-134A, RRID:AB_1720353), FOXM1 (Santa Cruz Biotechnology, sc-502, RRID:AB_631523), and CBP (Santa Cruz Biotechnology, sc-7300, RRID:AB_626817) antibodies were used for WB detection. In figure 1A and supplementary figure S2A, the same blot was probed with cyclin D1 and tubulin antibodies by cutting the membrane. In figure 2H, cyclin D1 and CBP blots correspond to the same membrane while E2F4 and FOXM1 blots correspond to an independent membrane. Image acquisition was performed with ImageQuant LAS 4000 mini (GE Healthcare). Image processing and quantification were performed with Multi Gauge software (Fujifilm). For qRT-PCR analysis, cDNA was generated from 1 µg RNA with qScript cDNA Synthesis kit (Quantabio). qRT–PCR reaction was performed using SYBR green (Roche). -

The Role of Genetics Mutations in Genes PORCN, TWIST2, HCCS In
ISSN: 2378-3672 Asadi and Jamali. Int J Immunol Immunother 2019, 6:038 DOI: 10.23937/2378-3672/1410038 Volume 6 | Issue 1 International Journal of Open Access Immunology and Immunotherapy REVIEW ARTICLE The Role of Genetics Mutations in Genes PORCN, TWIST2, HCCS in Goltz Syndrome Shahin Asadi1* and Mahsa Jamali2 Check for updates Division of Medical Genetics and Molecular Pathology Research, Molecular Genetics-IRAN-TABRIZ, Iran *Corresponding author: Shahin Asadi, Division of Medical Genetics and Molecular Pathology Research, Molecular Genetics-IRAN-TABRIZ, Iran are usually formed around the nose, the lips, the anal Abstract holes and the genitals of women with Goltz syndrome. Goltz syndrome (focal skin hypoplasia) is a genetic disorder In addition, papillomas may be present in the throat, that primarily affects the skin, skeletal system, eyes and face. People with Goltz syndrome have birth defects. These especially in the esophagus or larynx, and can cause disorders include very thin skin veins (skin hypoplasia), pink swallowing, respiration or sleep poisoning. The papillo- yellow nodules, subcutaneous fat, lack of upper skin layers mas can be removed if necessary by surgery from the (aplasia cutis), small clusters of superficial skin vessels growing regions. People with Goltz syndrome may have (telangiectasia), and veins in dark skin Or bright. Goltz syndrome is caused by mutation genes PORCN, TWIST2, small and abnormal nails on the toes. In addition, hair in HCCS. the scalp of these people can be weak or fragile or not [2] (Figure 2). Keywords Goltz syndrome, PORCN, TWIST2, HCCS genes, Skin and Many people with Goltz syndrome also have hand Skeletal disorders and foot disorders, including abnormalities such as oligodactyly in fingers and toes, fingers and legs (cin- Generalizations of Goltz Syndrome dactyly) or ecd rhodactyly. -

Pig Antibodies
Pig Antibodies gene_name sku Entry_Name Protein_Names Organism Length Identity CDX‐2 ARP31476_P050 D0V4H7_PIG Caudal type homeobox 2 (Fragment) Sus scrofa (Pig) 147 100.00% CDX‐2 ARP31476_P050 A7MAE3_PIG Caudal type homeobox transcription factor 2 (Fragment) Sus scrofa (Pig) 75 100.00% Tnnt3 ARP51286_P050 Q75NH3_PIG Troponin T fast skeletal muscle type Sus scrofa (Pig) 271 85.00% Tnnt3 ARP51286_P050 Q75NH2_PIG Troponin T fast skeletal muscle type Sus scrofa (Pig) 266 85.00% Tnnt3 ARP51286_P050 Q75NH1_PIG Troponin T fast skeletal muscle type Sus scrofa (Pig) 260 85.00% Tnnt3 ARP51286_P050 Q75NH0_PIG Troponin T fast skeletal muscle type Sus scrofa (Pig) 250 85.00% Tnnt3 ARP51286_P050 Q75NG8_PIG Troponin T fast skeletal muscle type Sus scrofa (Pig) 266 85.00% Tnnt3 ARP51286_P050 Q75NG7_PIG Troponin T fast skeletal muscle type Sus scrofa (Pig) 260 85.00% Tnnt3 ARP51286_P050 Q75NG6_PIG Troponin T fast skeletal muscle type Sus scrofa (Pig) 250 85.00% Tnnt3 ARP51286_P050 TNNT3_PIG Troponin T, fast skeletal muscle (TnTf) Sus scrofa (Pig) 271 85.00% ORF Names:PANDA_000462 EMBL EFB13877.1OrganismAiluropod High mobility group protein B2 (High mobility group protein a melanoleuca (Giant panda) ARP31939_P050 HMGB2_PIG 2) (HMG‐2) Sus scrofa (Pig) 210 100.00% Agpat5 ARP47429_P050 B8XTR3_PIG 1‐acylglycerol‐3‐phosphate O‐acyltransferase 5 Sus scrofa (Pig) 365 85.00% irf9 ARP31200_P050 Q29390_PIG Transcriptional regulator ISGF3 gamma subunit (Fragment) Sus scrofa (Pig) 57 100.00% irf9 ARP31200_P050 Q0GFA1_PIG Interferon regulatory factor 9 Sus scrofa (Pig) -

Based on Network Pharmacology Tools to Investigate the Molecular Mechanism of Cordyceps Sinensis on the Treatment of Diabetic Nephropathy
Hindawi Journal of Diabetes Research Volume 2021, Article ID 8891093, 12 pages https://doi.org/10.1155/2021/8891093 Research Article Based on Network Pharmacology Tools to Investigate the Molecular Mechanism of Cordyceps sinensis on the Treatment of Diabetic Nephropathy Yan Li,1 Lei Wang,2 Bojun Xu,1 Liangbin Zhao,1 Li Li,1 Keyang Xu,3 Anqi Tang,1 Shasha Zhou,1 Lu Song,1 Xiao Zhang,1 and Huakui Zhan 1 1Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, 610072 Sichuan, China 2Key Laboratory of Chinese Internal Medicine of Ministry of Education and Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing 100700, China 3Zhejiang Chinese Medical University, Hangzhou, 310053 Zhejiang, China Correspondence should be addressed to Huakui Zhan; [email protected] Received 27 August 2020; Revised 17 January 2021; Accepted 24 January 2021; Published 8 February 2021 Academic Editor: Michaelangela Barbieri Copyright © 2021 Yan Li et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Background. Diabetic nephropathy (DN) is one of the most common complications of diabetes mellitus and is a major cause of end- stage kidney disease. Cordyceps sinensis (Cordyceps, Dong Chong Xia Cao) is a widely applied ingredient for treating patients with DN in China, while the molecular mechanisms remain unclear. This study is aimed at revealing the therapeutic mechanisms of Cordyceps in DN by undertaking a network pharmacology analysis. Materials and Methods. In this study, active ingredients and associated target proteins of Cordyceps sinensis were obtained via Traditional Chinese Medicine Systems Pharmacology Database (TCMSP) and Swiss Target Prediction platform, then reconfirmed by using PubChem databases. -

A Uniform Human Wnt Expression Library Reveals a Shared Secretory Pathway and Unique Signaling Activities
Differentiation 84 (2012) 203–213 Contents lists available at SciVerse ScienceDirect Differentiation journal homepage: www.elsevier.com/locate/diff A uniform human Wnt expression library reveals a shared secretory pathway and unique signaling activities Rani Najdi a, Kyle Proffitt b, Stephanie Sprowl a, Simran Kaur b, Jia Yu b, Tracy M. Covey b, David M. Virshup b,1, Marian L. Waterman a,n a Department of Microbiology and Molecular Genetics, University of California, Irvine, CA, USA b Program in Cancer and Stem Cell Biology, Duke-NUS Graduate Medical School, Singapore article info abstract Article history: Wnt ligands are secreted morphogens that control multiple developmental processes during embry- Received 24 April 2012 ogenesis and adult homeostasis. A diverse set of receptors and signals have been linked to individual Accepted 16 June 2012 Wnts, but the lack of tools for comparative analysis has limited the ability to determine which of these Available online 9 July 2012 signals are general for the entire Wnt family, and which define subsets of differently acting ligands. We Keywords: have created a versatile Gateway library of clones for all 19 human Wnts. An analysis comparing Wnt signaling epitope-tagged and untagged versions of each ligand shows that despite their similar expression at the b-catenin mRNA level, Wnts exhibit considerable variation in stability, processing and secretion. At least 14 out of Wnt modification and secretion the 19 Wnts activate b-catenin-dependent signaling, an activity that is cell type-dependent and tracks with the stabilization of b-catenin and LRP6 phosphorylation. We find that the core Wnt modification and secretion proteins Porcupine (PORCN) and Wntless (WLS) are essential for all Wnts to signal through b-catenin-dependent and independent pathways. -

TET2 Binds the Androgen Receptor and Loss Is Associated with Prostate Cancer
Oncogene (2016), 1–12 © 2016 Macmillan Publishers Limited, part of Springer Nature. All rights reserved 0950-9232/16 www.nature.com/onc ORIGINAL ARTICLE TET2 binds the androgen receptor and loss is associated with prostate cancer ML Nickerson1,14, S Das2,KMIm3, S Turan1, SI Berndt4,HLi1,5,HLou1,5, SA Brodie4,6, JN Billaud7, T Zhang8, AJ Bouk4,6, D Butcher9, Z Wang4, L Sun10, K Misner1,WTan1,5, A Esnakula11, D Esposito12, WY Huang4, RN Hoover4, MA Tucker4, JR Keller10, J Boland4,6, K Brown8, SK Anderson1, LE Moore4, WB Isaacs13, SJ Chanock4, M Yeager4,6, M Dean1,14 and T Andresson2 Genetic alterations associated with prostate cancer (PCa) may be identified by sequencing metastatic tumour genomes to identify molecular markers at this lethal stage of disease. Previously, we characterized somatic alterations in metastatic tumours in the methylcytosine dioxygenase ten-eleven translocation 2 (TET2), which is altered in 5–15% of myeloid, kidney, colon and PCas. Genome-wide association studies previously identified non-coding risk variants associated with PCa and melanoma. We perform fine-mapping of PCa risk across TET2 using genotypes from the PEGASUS case-control cohort and identify six new risk variants in introns 1 and 2. Oligonucleotides containing two risk variants are bound by the transcription factor octamer-binding protein 1 (Oct1/POU2F1) and TET2 and Oct1 expression are positively correlated in prostate tumours. TET2 is expressed in normal prostate tissue and reduced in a subset of tumours from the Cancer Genome Atlas (TCGA). Small interfering RNA-mediated TET2 knockdown (KD) increases LNCaP cell proliferation, migration and wound healing, verifying loss drives a cancer phenotype. -

Tumour-Stroma Signalling in Cancer Cell Motility and Metastasis
Tumour-Stroma Signalling in Cancer Cell Motility and Metastasis by Valbona Luga A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy, Department of Molecular Genetics, University of Toronto © Copyright by Valbona Luga, 2013 Tumour-Stroma Signalling in Cancer Cell Motility and Metastasis Valbona Luga Doctor of Philosophy Department of Molecular Genetics University of Toronto 2013 Abstract The tumour-associated stroma, consisting of fibroblasts, inflammatory cells, vasculature and extracellular matrix proteins, plays a critical role in tumour growth, but how it regulates cancer cell migration and metastasis is poorly understood. The Wnt-planar cell polarity (PCP) pathway regulates convergent extension movements in vertebrate development. However, it is unclear whether this pathway also functions in cancer cell migration. In addition, the factors that mobilize long-range signalling of Wnt morphogens, which are tightly associated with the plasma membrane, have yet to be completely characterized. Here, I show that fibroblasts secrete membrane microvesicles of endocytic origin, termed exosomes, which promote tumour cell protrusive activity, motility and metastasis via the exosome component Cd81. In addition, I demonstrate that fibroblast exosomes activate autocrine Wnt-PCP signalling in breast cancer cells as detected by the association of Wnt with Fzd receptors and the asymmetric distribution of Fzd-Dvl and Vangl-Pk complexes in exosome-stimulated cancer cell protrusive structures. Moreover, I show that Pk expression in breast cancer cells is essential for fibroblast-stimulated cancer cell metastasis. Lastly, I reveal that trafficking in cancer cells promotes tethering of autocrine Wnt11 to fibroblast exosomes. These studies further our understanding of the role of ii the tumour-associated stroma in cancer metastasis and bring us closer to a more targeted approach for the treatment of cancer spread. -
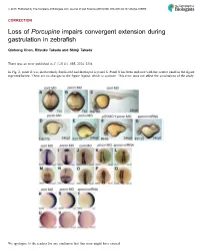
Porcupine Impairs Convergent Extension During Gastrulation in Zebrafish
ß 2015. Published by The Company of Biologists Ltd | Journal of Cell Science (2015) 000, 000–000 doi:10.1242/jcs.168989 CORRECTION Loss of Porcupine impairs convergent extension during gastrulation in zebrafish Qiuhong Chen, Ritsuko Takada and Shinji Takada There was an error published in J. Cell Sci. 125, 2224–2234. In Fig. 2, panel R was inadvertently duplicated and displayed as panel S. Panel S has been replaced with the correct panel in the figure reprinted below. There are no changes to the figure legend, which is accurate. This error does not affect the conclusions of the study. We apologise to the readers for any confusion that this error might have caused. 1 Journal of Cell Science JCS168989.3d 22/1/15 10:57:22 The Charlesworth Group, Wakefield +44(0)1924 369598 - Rev 9.0.225/W (Oct 13 2006) 2224 Research Article Loss of Porcupine impairs convergent extension during gastrulation in zebrafish Qiuhong Chen1, Ritsuko Takada1 and Shinji Takada1,2,* 1Okazaki Institute for Integrative Bioscience and National Institute for Basic Biology, National Institutes of Natural Sciences, Okazaki, Aichi 444-8787, Japan 2Department of Molecular Biomechanics, The Graduate University for Advanced Studies (SOKENDAI), Okazaki, Aichi 444-8787, Japan *Author for correspondence ([email protected]) Accepted 9 January 2012 Journal of Cell Science 125, 2224–2234 ß 2012. Published by The Company of Biologists Ltd doi: 10.1242/jcs.098368 Summary Porcupine (Porcn), an O-acyltransferase located in the endoplasmic reticulum (ER), is required for lipidation of Wnt proteins to enable their trafficking from the ER in mammalian cell culture. -

Microrna-124-3P Suppresses Mouse Lip Mesenchymal Cell Proliferation
Suzuki et al. BMC Genomics (2019) 20:852 https://doi.org/10.1186/s12864-019-6238-4 RESEARCH ARTICLE Open Access MicroRNA-124-3p suppresses mouse lip mesenchymal cell proliferation through the regulation of genes associated with cleft lip in the mouse Akiko Suzuki1,2, Hiroki Yoshioka1,2, Dima Summakia1, Neha G. Desai1,3, Goo Jun3,4, Peilin Jia5, David S. Loose4,6, Kenichi Ogata1,2, Mona V. Gajera1,3, Zhongming Zhao3,4,5 and Junichi Iwata1,2,4* Abstract Background: Cleft lip (CL), one of the most common congenital birth defects, shows considerable geographic and ethnic variation, with contribution of both genetic and environmental factors. Mouse genetic studies have identified several CL-associated genes. However, it remains elusive how these CL-associated genes are regulated and involved in CL. Environmental factors may regulate these genes at the post-transcriptional level through the regulation of non-coding microRNAs (miRNAs). In this study, we sought to identify miRNAs associated with CL in mice. Results: Through a systematic literature review and a Mouse Genome Informatics (MGI) database search, we identified 55 genes that were associated with CL in mice. Subsequent bioinformatic analysis of these genes predicted that a total of 33 miRNAs target multiple CL-associated genes, with 20 CL-associated genes being potentially regulated by multiple miRNAs. To experimentally validate miRNA function in cell proliferation, we conducted cell proliferation/viability assays for the selected five candidate miRNAs (miR-124-3p, let-7a-5p, let-7b-5p, let-7c-5p, and let-7d-5p). Overexpression of miR-124-3p, but not of the others, inhibited cell proliferation through suppression of CL-associated genes in cultured mouse embryonic lip mesenchymal cells (MELM cells) isolated from the developing mouse lip region.