Genetic Structure and Connectivity of the Endangered Butler's Gartersnake (Thamnophis Butleri) Across the Fragmented Landscape
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Essex County, Massachusetts, 1630-1768 Harold Arthur Pinkham Jr
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Winter 1980 THE TRANSPLANTATION AND TRANSFORMATION OF THE ENGLISH SHIRE IN AMERICA: ESSEX COUNTY, MASSACHUSETTS, 1630-1768 HAROLD ARTHUR PINKHAM JR. University of New Hampshire, Durham Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation PINKHAM, HAROLD ARTHUR JR., "THE TRANSPLANTATION AND TRANSFORMATION OF THE ENGLISH SHIRE IN AMERICA: ESSEX COUNTY, MASSACHUSETTS, 1630-1768" (1980). Doctoral Dissertations. 2327. https://scholars.unh.edu/dissertation/2327 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. INFORMATION TO USERS This was produced from a copy of a document sent to us for microfilming. Whfle the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help you understand markings or notations vhich may appear on this reproduction. 1. The sign or “target” for pages apparently lacking from the document photographed is “Missing Page(s)”. If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure you of complete continuity. 2. When an image on the film is obliterated with a round black mark it is an indication that the film inspector noticed either blurred copy because of movement during exposure, or duplicate copy. -

Property Tax Information
Commonwealth of Massachusetts Middlesex North Registry of Deeds 360 Gorham Street Lowell, MA 01852 www.lowelldeeds.com Richard P. Howe Jr. Tel. (978) 322-9000 Register of Deeds Fax. (978) 322-9001 Property Tax Information To obtain information about property taxes, you must call the Tax Collector or Treasurer of the town where the property is located. In Massachusetts, neither the county nor the Registry of Deeds has any property tax information. The following pages contain an alphabetical listing of all of the cities and towns in Massachusetts, (what we think is) the telephone number to call for information about property taxes, and the name of the Registry of Deeds in which records for that town are located. Town Telephone Ext Registry District Abington (781) 982-2131 Plymouth Acton (978) 263-9107 Middlesex South Acushnet (508) 998-0212 Bristol South Adams (413) 743-8390 Berkshire North Agawam (413) 786-0400 Ext 220 Hampden Alford (413) 528-4536 Berkshire South Allston (617) 635-3327 Suffolk Amesbury (978) 388-8105 Essex South Amherst (413) 856-4020 Hampshire Andover (978) 623-8249 Essex North Arlington (781) 316-3030 Middlesex South Ashburnham (978) 827-4102 Worcester North Ashby (978) 386-2427 Middlesex South Ashfield (413) 628-4428 Franklin Ashland (508) 881-0107 Middlesex South Athol (978) 249-8484 Worcester South Attleboro (508) 223-2222 Ext 3126 Bristol North Auburn (508) 832-7705 Worcester South Avon (508) 588-0141 Norfolk Ayer (978) 772-8215 Middlesex South Barnstable (508) 862-4054 Barnstable Barre (978) 355-5001 Worcester -

Official Records
SECTION B JUNE 19, 2017 BANKER & TRADESMAN Official Records MASSACHUSETTS MARKET STATISTICS INDEX Volume of Mortgages County Sales Charts In This Week’s Issue for Single-Family Homes None 6000$6,0006000 Both Both 5000 Real Estate Records 5000$5,000 Renance Renance 4000 Purchase PAGE COUNTY TRANSACTIONS THRU PAGE COUNTY TRANSACTIONS THRU 4000$4,0003000 B2 SuffolkPurchase ........ 06/02/17 B14 Franklin ....... 06/02/17 2000 $3,000 B6 Barnstable ..... 06/02/17 B15 Hampden ...... 06/02/17 3000 1000 0 B7 Berkshire Middle 06/02/17 B17 Hampshire ..... 06/02/17 2000$2,000 Jun Jul Aug Sep Oct Nov Dec Jan Feb Mar Apr May Jun B8 Berkshire North .06/02/17 B17 Middlesex North. 06/02/17 $1,000 1000 B8 Berkshire South .06/02/17 B19 Middlesex South. 06/02/17 0$0 B8 Bristol Fall River 06/02/17 B24 Nantucket ...... 06/02/17 JunMay JulJun. AuJul.g SeAug.p OcSept.t NoOct.v DeNov.c JanDec. FeJan.b MaMayr ApMar.r MaApr.y JuMayn ’16 ’17 B9 Bristol North ... 06/02/17 B24 Norfolk ........ 06/02/17 B10 Bristol South ... 06/02/17 B27 Plymouth ...... 06/02/17 6600 $6,600 BothMonth Purchase Refinance Both 6600 Both B11 Dukes ......... 06/02/17 B30 Worcester ...... 06/02/17 5500 5500 $5,500 Renance Renance 4400 Purchase B11 Essex North .... 06/02/17 B34 Worcester North. 06/02/17 $4,4004400 Purchase 3300 May 2013 $1,399 $4,236 $5,636 B12 Essex South .... 06/02/17 2200 $3,3003300 May 2014 $1,394 $1,392 $2,786 1100 0 2200 May 2015 $1,335 $2,573 $3,908 Fe$2,200b. -

The Canadian Parliamentary Guide
NUNC COGNOSCO EX PARTE THOMAS J. BATA LI BRARY TRENT UNIVERSITY us*<•-« m*.•• ■Jt ,.v<4■■ L V ?' V t - ji: '^gj r ", •W* ~ %- A V- v v; _ •S I- - j*. v \jrfK'V' V ■' * ' ’ ' • ’ ,;i- % »v • > ». --■ : * *S~ ' iJM ' ' ~ : .*H V V* ,-l *» %■? BE ! Ji®». ' »- ■ •:?■, M •* ^ a* r • * «'•# ^ fc -: fs , I v ., V', ■ s> f ** - l' %% .- . **» f-•" . ^ t « , -v ' *$W ...*>v■; « '.3* , c - ■ : \, , ?>?>*)■#! ^ - ••• . ". y(.J, ■- : V.r 4i .» ^ -A*.5- m “ * a vv> w* W,3^. | -**■ , • * * v v'*- ■ ■ !\ . •* 4fr > ,S<P As 5 - _A 4M ,' € - ! „■:' V, ' ' ?**■- i.." ft 1 • X- \ A M .-V O' A ■v ; ■ P \k trf* > i iwr ^.. i - "M - . v •?*»-• -£-. , v 4’ >j- . *•. , V j,r i 'V - • v *? ■ •.,, ;<0 / ^ . ■'■ ■ ,;• v ,< */ ■" /1 ■* * *-+ ijf . ^--v- % 'v-a <&, A * , % -*£, - ^-S*.' J >* •> *' m' . -S' ?v * ... ‘ *•*. * V .■1 *-.«,»'• ■ 1**4. * r- * r J-' ; • * “ »- *' ;> • * arr ■ v * v- > A '* f ' & w, HSi.-V‘ - .'">4-., '4 -' */ ' -',4 - %;. '* JS- •-*. - -4, r ; •'ii - ■.> ¥?<* K V' V ;' v ••: # * r * \'. V-*, >. • s s •*•’ . “ i"*■% * % «. V-- v '*7. : '""•' V v *rs -*• * * 3«f ' <1k% ’fc. s' ^ * ' .W? ,>• ■ V- £ •- .' . $r. « • ,/ ••<*' . ; > -., r;- •■ •',S B. ' F *. ^ , »» v> ' ' •' ' a *' >, f'- \ r ■* * is #* ■ .. n 'K ^ XV 3TVX’ ■■i ■% t'' ■ T-. / .a- ■ '£■ a« .v * tB• f ; a' a :-w;' 1 M! : J • V ^ ’ •' ■ S ii 4 » 4^4•M v vnU :^3£'" ^ v .’'A It/-''-- V. - ;ii. : . - 4 '. ■ ti *%?'% fc ' i * ■ , fc ' THE CANADIAN PARLIAMENTARY GUIDE AND WORK OF GENERAL REFERENCE I9OI FOR CANADA, THE PROVINCES, AND NORTHWEST TERRITORIES (Published with the Patronage of The Parliament of Canada) Containing Election Returns, Eists and Sketches of Members, Cabinets of the U.K., U.S., and Canada, Governments and Eegisla- TURES OF ALL THE PROVINCES, Census Returns, Etc. -

Retooling for a Prosperous Ontario
Retooling for a Prosperous Ontario A GLOBAL PERSPECTIVE ON SKILLED TRADES Retooling for a Prosperous Ontario A GLOBAL PERSPECTIVE ON SKILLED TRADES OCTOBER 2006 Table of Contents Executive Summary 3 Introduction 5 SECTION I: ONTARIO 9 Ontario’s Challenges 9 Ontario’s Investment 2 Ontario’s Apprenticeship Program 4 SECTION II: ENVIRONMENTAL SCAN OF OTHER JURISDICTIONS 7 Australia 7 United Kingdom 20 Germany 2 Alberta 26 Manitoba 28 SECTION III: SUMMARY & CONCLUSION 3 APPENDIX I 35 APPENDIX II 37 APPENDIX III 4 RETOOLING FOR A PROSPEROUS ONTARIO Executive Summary This report is an environmental scan of “best practices” from other jurisdictions in Canada and around the world relating to apprenticeship training. The jurisdictions selected in this report represent sources of ideas and strategies that, given a thorough analysis and evaluation, could be successfully applied in Ontario. Ontario has several outstanding programs in place to help address the skilled trades shortage. However, individuals are often unaware of the programs and incentives that currently exist in Ontario nor are they aware of how to access information on such programs. Our research indicates that the current challenges Ontario faces in regards to apprenticeship training and the skilled labour shortage are similar to those experienced in many other jurisdictions. A few examples of these challenges are: . a negative perception associated with a career in a skilled trade; . a lack of awareness; . a hesitation from employers to train an apprentice due to training costs and “poaching”. When reviewing “best practices” in other jurisdictions, several themes are evident when analyzing these successful ideas and strategies. These “themes” can be broken down into the following: . -

The Politico's Guide to Electoral Reform in Britain
Patrick Dunleavy, Helen Margetts and Stuart Weir The Politico's guide to electoral reform in Britain Book section Original citation: Originally published in Dunleavy, Patrick, Margetts, Helen and Weir, Stuart (1998) The Politico's guide to electoral reform in Britain. Politico's Publishing, London, UK. ISBN 190230120X © Democratic Audit This version available at: http://eprints.lse.ac.uk/62253/ Available in LSE Research Online: June 2015 LSE has developed LSE Research Online so that users may access research output of the School. Copyright © and Moral Rights for the papers on this site are retained by the individual authors and/or other copyright owners. Users may download and/or print one copy of any article(s) in LSE Research Online to facilitate their private study or for non-commercial research. You may not engage in further distribution of the material or use it for any profit-making activities or any commercial gain. You may freely distribute the URL (http://eprints.lse.ac.uk) of the LSE Research Online website. the Guide to ELECTORAL REFORM in Britain Patrick Dunleavy, Helen Margetts and Stuart Weir First published in Great Britain 1998 by Politico’s Publishing 8 Artillery Row London SW1P 1RZ England Telephone 0171 931 0090 Email [email protected] Website http://www.politicos.co.uk Copyright Patrick Dunleavy, Helen Margetts and Stuart Weir 1998 The right of Patrick Dunleavy, Helen Margetts and Stuart Weir to be identified as authors of this work has been asserted by them in accordance with the Copyright, Design and Patents Act 1988 A catalogue record for this book is available from the British library ISBN 190230120X Printed and bound in Great Britain by Colourworks Typesetting and cover design by Tony Garrett All rights reserved. -
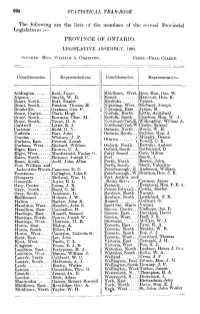
694 STATISTICAL YEAR-BOOK the Following Are the Lists of The
694 STATISTICAL YEAR-BOOK The following are the lists of the members of the several Provincial Legislatures :— PROVINCE OF ONTARIO. LEGJSLATIVE ASSEMBLY, 1903. SPEAKER—Hox. WILLIAM A. CHARLTON. CLEKK—CHAS. CLARKE. Constituencies. Representatives. Constituencies, Representatives. Addington Reid, James Middlesex, West. Ross, Hon. Geo. W. Algoma Smyth, W. R. Monck Harconrt, Hon. R. Brant, North Burt, Daniel Muskoka Vacant. Brant, South Preston, Thomas H. Nipissing, West.. Michaud, Joseph Brockville Graham, Geo. P. Ni pissing, East.. James, M. Bruce, Centre.... Clark, Hugh Norfolk, North .. Little, Archibald Bruce, North Bowman, Chas. M. Norfolk, South. Charlton, Hon. W. A. Bruce, South Truax, R. A. NorthumbTnd,E. Wilkmghby, William A. Cardwell Little, E. A. Northumb'l'nd, W Clarke, Samuel Carleton Kidd, G. N. Ontario, North .. Hoyle, W. H. Dufferin Barr, John Ontario, South... Dryden, Hon. J. Dundas Whitney, J. P. f Murphy, Dennis Durham, East.... Preston, Josiah Ottawa. Powell, C. B. Durham, West... Rickard, William Oxford, North... Pattullo, Andrew Elgin, East Brower, C. A. Oxford, South.... Sutherland, D. Elgin, West Macdiarmid, Finlay G. Parry Sound Carr, Milton Essex, North Reaunie, Joseph C. Peel Smith, J. Essex, South Auld. John Allan Perth, North .... Brown, John. Fort William and Perth, South Stock, Valentine Lake of the Woods Cameron, D. C. Peterborough, E. Anderson, William. Frontenac Gallagher, John S. Peterborough, W. Stratton,Hon. J. R. Glengarry McLeod, Wm. D. Port Arthnr and Grenville Joynt, R. L. Rainy River ... Conmee, James Grey, Centre Lucas, J. B. Prescott Evanturel, Hon. F. E. A. Grey, North Boyd, G. M. Prince Edward... Currie, Morley Grey. South Jamieson, D. Renfrew, North.. Vacant. -

THE RETAIL SALES TAX: an ECONOMIC STUDY by KENYON E
THE RETAIL SALES TAX: AN ECONOMIC STUDY by KENYON E. POOLE A study prepared for The Ontario Committee on Taxation THE RETAIL SALES TAX: AN ECONOMIC STUDY by KENYON E. POOLE, Ph.D. A study prepared for The Ontario Committee on Taxation Printed and Published by FRANK FOGG Queen’s Printer Preface TH E present study was submitted at the beginning of 1964. Much has happened ■ since then with respect to all systems of taxation, including those of the Canadian provinces and the American states. Time limitations have prevented bringing these data up to 1968. The author would like, moreover, to take this opportunity to emphasize that his favourable view with respect to the sales tax is based on two considerations. First, the sales tax is only one tax in the revenue system as a whole. Second, exemptions can be provided for food, clothing, etc., which make the tax at least proportional in the first several thousand dollars of income and spending. In a recommendation in favour of a sales tax there is no implication that any desired use may not be made of a progressive income tax, a graduated inheritance tax, or even a graduated net worth tax. The several taxes can be combined in such a way as to satisfy the public’s view on the desired after-tax distribution of income and wealth. The author’s thanks are gladly given to those who contributed to the study at key points along the line of its development Robert Evans is due special thanks for preparing the first two chapters. -

The Association of Professional Archaeologists Newsletter
The Association of Professional Archaeologists Newsletter 2013-02 WINTER EDITION Web Page: www.apaontario.ca Facebook: www.facebookcom/APAOntario Your New Executive Directors Archaeological Society in 2007. She has worked with • President, Sue Bazely numerous heritage organizations and has identified • Vice President, Keith Powers broadening the scope of representation as her key aim • Past President, Scarlett Janusas for the APA. • Director, Membership and Webmaster, Keith Powers has conducted archaeological fieldwork James B Bandow in Ontario for over 15 years. He received formal • Director, First Nations, Laurie Jackson training in archaeological fieldwork through the Boyd • Director, Northern Ontario, Scott Archaeological Field School at the Seed-Barker site. Hamilton Keith received academic training in archaeology • Director, Investigations, Carla Parslow through York University and the University of Toronto. • Director, Field Director, Jeff Muir Keith completed his graduate work at the University of • Director, Innovations and Process, York, England where his research focused on the Douglas Yahn application of geophysical techniques to Iroquoian archaeology. Other Members Since 2002 he has been the Principal Archaeologist of • Treasurer, Cathy Crinnion The Archaeologists Inc. and has conducted hundreds of archaeological assessments within the province. He • Secretary , Open continues to pursue research interests in the use of • Newsletter, Tom Arnold geophysical techniques in archaeological investigations and has applied these techniques to a variety of sites Executive Bios including Iroquoian villages, historic homesteads, and cemeteries. Sue Bazely has an MA in Archaeology and Heritage from the University of Leicester (UK) and BA in Keith has worked on archaeological projects Anthropology (Archaeology) from the University of throughout Canada and worldwide including sites in Toronto. -

ECPA Review May 2015
‐ Innovative Research Group, Inc. Toronto • Vancouver Consumer Consultation Report Energy Consumer Protection Act Review May 2015 Prepared for: Consumer Consultation Report ECPA Review May 2015 This report has been prepared by Innovative Research Group Inc. (INNOVATIVE) for the Ontario Energy Board. The conclusions drawn and opinions expressed are those of the authors. Innovative Research Group Inc. 56 The Esplanade, Suite 310 Toronto ON | M5E 1A7 Tel: 416.642.6340 Fax: 416.640.5988 www.innovativeresearch.ca Contents Introduction ................................................................................................................................................... 1 Consultation Process Overview ............................................................................................ 1 Executive Summary .............................................................................................................. 5 Exploratory Findings .................................................................................................................................. 5 How well is the ECPA working in theory? ................................................................................................... 6 How well is the ECPA working in practice? ................................................................................................. 9 Literature Review ........................................................................................................................................ 12 Implications for the ECPA -

Electoral Districts, Voters on List and Votes Polled, Names and Addresses of Members of the House of Commons, As Elected at the Nineteenth General Election, Mar
PARLIAMENTARY REPRESENTATION 69 9.—Electoral Districts, Voters on List and Votes Polled, Names and Addresses of Members of the House of Commons, as Elected at the Nineteenth General Election, Mar. 26, 1940—continued. Province and Popula Voters Votes Party Electoral District tion, on Polled Name of Member Affili P.O. Address 1931 List ation No. No. No. Quebec—concluded Montreal Island—cone St. Henry 78,127 46,236 31,282 BONNIEK, J. A. Lib. Montreal, Que. St. James 89,374 64,823 35,587 DuROCHER, E. .. Lib. Montreal, Que. St. Lawrence- St. George 40,213 29,416 18,544 CLAXTON, B Lib. Montreal, Que. St. Mary 77,472 49,874 30,289 DESLAURIERS, H1 Lib. Montreal, Que. Verdun 63,144 40,555 28,033 COTE, P. E Lib. Verdun, Que. Ontario— (82 members) Algoma East... 27,925 15,250 10,386 FARQUHAR, T. Lib. Mindemoya, Ont. Algoma West... 35,618 22,454 16,580 NIXON, G. E.. Lib. Sault Ste. Marie, Ont. Brant 21,202 12,980 9,229 WOOD, G. E Lib... Cainsville, Ont. Brantford City.. 32,274 21,607 15,762 MACDONALD, W. R Lib... Brantford, Ont. Bruce 29,842 19,359 12,781 TOMLINSON, W. R Lib... Port Elgin, Ont. Carleton 31,305 20,716 14,481 HYNDMAN, A. B.2 Cons. Carp, Ont. Cochrane 58,284 44,559 26,729 BRADETTE, J. A Lib... Cochrane, Ont. Dufferin-Simcoe. 27,394 19,338 10,840 ROWE, Hon. W. E.... Cons. Newton Robinson, Ont. Durham 25,782 17,095 12,254 RlCKARD, W. F Lib Newcastle, Ont. Elgin 43,436 30,216 20,902 MILLS, W. -

Legislators and Legislatures of Ontario : a Reference Guide
Msktor^s Ijegisl/itivcUkmy isktors a ofOntum a tvfenmceguide n/oCume 4/1984-1991 dt m m. Ontario Le^sktiveL3mt Canadian Cataloguing in Publication Data Forman, Debra, 1956- Legislators and legislatures of Ontario 4. 1984-1991. Contents: v. 1. 1792-1866. - v. 2. 1867-1929. ~ v. 3. 1930-1984. - v. - 0-7743- ISBN 0-7743-9021-2 (set). - 0-7743-9022-0 (v. 1). - 0-7743-9023-9 (v. 2). 9024-7 (v. 3). - 0-7729-9328-9 (v. 4). 3. 1. Ontario. Legislative Assembly-History. 2. Ontario-Politics and government. Legislators-Ontario-History. I. Ontario. Legislative Library. II. Title. JL273.F6 1984 328.713'09 84-093008-9 1 1 Contents Foreword v Introduction vii Errata viii General Elections 1984-1991 1 Presidentsof the Executive Council 1984-1991 2 Alphabetical Index of Members 1792- 1991 3-27 Executive CouncUs of Ontario 1 984- 1 99 29-5 Death Notices Former Ontario MPPs 1984-1991 53-54 32nd Legislature 55-87 33rd Legislature 88-109 34th Legislature 110-181 35th Legislature 182-201 ® IV Foreword Approximately 5,200 men and women have served as Members of the House of Assembly of the Province of Upper Canada (1792-1841), as Members from Canada West in the Legislative Assembly of the united Province of Canada (1841-1867), and as Members of the legislative Assembly of the Province of Ontario (1867-1991). In the past, identification or verification of a particular Member, Legislature or Electoral District has proved to be a tedious and time-consuming task for those researching Ontario's political history because the information is scattered in a great many sources.