Addis Ababa University, Graduate Programs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
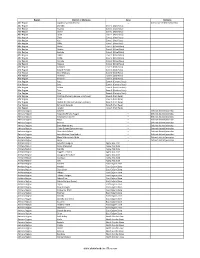
Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

ETHIOPIA Humanitarian Access Situation Report June – July 2019
ETHIOPIA Humanitarian Access Situation Report June – July 2019 This report is produced by OCHA Ethiopia in collaboration with humanitarian partners. It covers the period June - July 2019. The next report will be issued around September - October 2019. OVERVIEW IUS • In June - July, Ethiopia experienced an at- TIGRAY 276 Access incidents reported tempted government overthrow in Amhara, Western socio-political unrest in Sidama (SNNPR), North Gondar Wag Hamra Central Gondar and a rise in security incidents in Southwest- Zone 4 (Fantana Rasu) AFAR ern Oromia and Gambella. The quality of ac- Zone 1 (Awsi Rasu) cess declined, limiting assistance to people AMHARA No. o incidents by one South Wello Metekel in need, against a backdrop of massive gov- Oromia East Gojam BENISHANGUL Zone 5 (Hari Rasu) 4 13 35 49 AsosaGUMUZ Siti ernment-led returns of IDP to areas of origin. Zone 3 (Gabi Rasu) North Shewa(O) North Shewa(A) Kemashi Dire Dawa urban West Wellega East Wellega DIRE DAWA West Shewa Fafan • Hostilities between Ethiopian Defense Forc- ADDIS ABABA Kelem Wellega East Hararge Finfine Special West Hararge es (EDF) and Unidentified Armed Groups Buno Bedele East Shewa Etang Special Ilu Aba Bora Jarar OROMIA Erer (UAGs) as well as inter-ethnic, remained the GAMBELA Jimma Agnewak main access obstacle, with 197 incidents Doolo Nogob West Arsi SOMALI (out of 276), mostly in Southwestern Oromia SNNP Sidama Bale Korahe (110). The Wellegas, West Guji (Oromia), and Gedeo Shabelle Gambella, were the most insecure areas for Segen Area P. West Guji Guji aid workers. Liban Borena • In June, conflict in the Wellegas scaled up, Daawa with explosive devices attacks causing ci- Source: Access Incidents database vilian casualties in urban centres. -

List of Zones of Ethiopia
Region Zone Addis Ababa Addis Ababa Afar Region Administrative Zone 1 (a.k.a. Awsi Rasu) Afar Region Administrative Zone 2 (a.k.a. Kilbet Rasu) Afar Region Administrative Zone 3 (a.k.a. Gabi Rasu) Afar Region Administrative Zone 4 (a.k.a. Fantena Rasu) Afar Region Administrative Zone 5 (a.k.a. Hari Rasu) Afar Region Argobba (special woreda) Amhara Region Agew Awi Amhara Region East Gojjam Amhara Region North Gondar Amhara Region North Shewa Amhara Region North Wollo Amhara Region Oromia Amhara Region South Gondar Amhara Region South Wollo Amhara Region Wag Hemra Amhara Region West Gojjam Amhara Region Bahir Dar (special zone) Benishangul-Gumuz Region Asosa Benishangul-Gumuz Region Kamashi Benishangul-Gumuz Region Metekel Dire Dawa Dire Dawa Gambela Region Anuak Gambela Region Mezhenger Gambela Region Nuer Harari Region Harari Oromia Region Arsi Oromia Region Bale Oromia Region Borena Oromia Region East Hararghe Oromia Region East Shewa Oromia Region East Welega Oromia Region Guji Oromia Region Horo Gudru Welega Oromia Region Illubabor Oromia Region Jimma Oromia Region Kelem Welega Oromia Region North Shewa Oromia Region South West Shewa Oromia Region West Arsi Oromia Region West Hararghe Oromia Region West Shewa Oromia Region West Welega Oromia Region Adama (special zone) Oromia Region Jimma (special zone) Oromia Region Oromia-Finfinne (special zone) www.downloadexcelfiles.com Somali Region Afder Somali Region Degehabur Somali Region Fiq Somali Region Gode Somali Region Jijiga Somali Region Korahe Somali Region Liben Somali Region Shinile -

Ethiopia: Administrative Map (August 2017)
Ethiopia: Administrative map (August 2017) ERITREA National capital P Erob Tahtay Adiyabo Regional capital Gulomekeda Laelay Adiyabo Mereb Leke Ahferom Red Sea Humera Adigrat ! ! Dalul ! Adwa Ganta Afeshum Aksum Saesie Tsaedaemba Shire Indasilase ! Zonal Capital ! North West TigrayTahtay KoraroTahtay Maychew Eastern Tigray Kafta Humera Laelay Maychew Werei Leke TIGRAY Asgede Tsimbila Central Tigray Hawzen Medebay Zana Koneba Naeder Adet Berahile Region boundary Atsbi Wenberta Western Tigray Kelete Awelallo Welkait Kola Temben Tselemti Degua Temben Mekele Zone boundary Tanqua Abergele P Zone 2 (Kilbet Rasu) Tsegede Tselemt Mekele Town Special Enderta Afdera Addi Arekay South East Ab Ala Tsegede Mirab Armacho Beyeda Woreda boundary Debark Erebti SUDAN Hintalo Wejirat Saharti Samre Tach Armacho Abergele Sanja ! Dabat Janamora Megale Bidu Alaje Sahla Addis Ababa Ziquala Maychew ! Wegera Metema Lay Armacho Wag Himra Endamehoni Raya Azebo North Gondar Gonder ! Sekota Teru Afar Chilga Southern Tigray Gonder City Adm. Yalo East Belesa Ofla West Belesa Kurri Dehana Dembia Gonder Zuria Alamata Gaz Gibla Zone 4 (Fantana Rasu ) Elidar Amhara Gelegu Quara ! Takusa Ebenat Gulina Bugna Awra Libo Kemkem Kobo Gidan Lasta Benishangul Gumuz North Wello AFAR Alfa Zone 1(Awsi Rasu) Debre Tabor Ewa ! Fogera Farta Lay Gayint Semera Meket Guba Lafto DPubti DJIBOUTI Jawi South Gondar Dire Dawa Semen Achefer East Esite Chifra Bahir Dar Wadla Delanta Habru Asayita P Tach Gayint ! Bahir Dar City Adm. Aysaita Guba AMHARA Dera Ambasel Debub Achefer Bahirdar Zuria Dawunt Worebabu Gambela Dangura West Esite Gulf of Aden Mecha Adaa'r Mile Pawe Special Simada Thehulederie Kutaber Dangila Yilmana Densa Afambo Mekdela Tenta Awi Dessie Bati Hulet Ej Enese ! Hareri Sayint Dessie City Adm. -

Enabling Successful Migration for Youth in Addis Ababa Quantitative Survey
Enabling Successful Migration for Youth in Addis Ababa Quantitative Survey Z1 Respondent Code Sequential number according to order of the respondent (101, 102, 103, etc.) Z2 Interviewer Code Z3 Date of Interview (DD/MM/YY) / / 1 8 Z4 Location Code Based on site Z5 Start time of Interview (according to Western clock) : CONSENT: Did you complete the entire verbal script for consent, answer all questions from the respondent, and and receive a fully informed consent to participate? Investigator name: Investigator Signature: Are you willing to go ahead? Yes → continue to A1 No → stop the survey, thank the respondent, and approach another potential respondent 1 I. DEMOGRAPHIC CHARACTERISTICS A1 How old are you? Enter age A2 Sex 0 = male 1 = female 8 = don’t know 9 = refused to answer A3 Where in Ethiopia are you from? Enter code for region Enter code for zone Enter name of woreda Enter name of kebele A4 Where in Addis Ababa do you live? Enter code for subcity Enter name of district A5 Which year did you first arrive in Enter year (according to Western Addis? calendar, not Ethiopian) A6 How much time (cumulative) have Enter total number of months you spent in Addis Ababa? A7 What is your marital status? 1 = married 2 = engaged, 3 = in a partnership 4 = single 5 = widowed 6 = divorced/separated 8 = don’t know 9 = refused to answer A8 Do you have any children? 0 = no 1 = yes 8 = don’t know 9 = refused to answer A9 What is your religion? 1 = Ethiopian Orthodox 2 = Muslim 3 = Protestant 4 = Traditional/local 5 = Catholic 6 = Other 8 = Don’t know -

Ethiopia: Humanitarian Access Situation Report
ETHIOPIA Humanitarian Access Situation Report January - March 2020 This report is produced by OCHA Ethiopia in collaboration with humanitarian partners. It covers the period January to March 2020. The next report will be issued in June 2020. OVERVIEW • The operational environment to relief operations North Number of incidents by woreda Western Central remained permissive through the reporting period. Western TIGRAY Eastern 1 - 2 3 - 4 5 - 6 South Kilbet Most access impediments continue related to hos- North Rasu Gondar Eastern Wag tilities, intra-community conflicts or social unrest, Central Southern Gondar Hamra West Fantana hindering the quality of the humanitarian response, Gondar AMHARA North Wello Rasu AFAR and to COVID-19. South Awsi Gondar Rasu Metekel Hari Awi West East South Wello Gojam Rasu • Humanitarian partners are committed to support BENISHANGUL Gojam Oromia GUMUZ Siti the government response to COVID-19 and ensure North North Gabi Kemashi Horo Shewa DIRE DAWA West Shewa Rasu that critical activities are sustained. Partners are Gudru West Mao Komo Wellega Wellega Shewa Fafan Special East Addis Ababa actively implementing precautionary measures to Wellega HARARI Kelem Wellega East South West West ensure the safety of aid personnel and the popula- Buno Bedele East Hararge Hararge Ilu Aba Shewa Shewa Guraghe GAMBELA Bora Jarar tion. Nuwer Arsi Erer Agnewak Jimma Hadiya Siltie Sheka Yem Sp.Halaba Sp. OROMIA Kembata Mejenger Kefa Doolo Dawuro Tibaro Nogob SOMALI • The humanitarian community is working with gov- Bench Maji West Arsi Konta Sp. Wolayita Bale Gofa Sidama ernment counterparts to ensure that partners can Gamo Korahe Mirab Basketo Gedeo continue movements and operations throughout Omo Amaro SNNP Derashe Alle Guji Shabelle the country, bearing in mind restrictions to contain South Omo BurjiWest Guji Konso Afder the spread of COVID-19. -

Ethiopia Access Snapshot - Afar Region and Siti Zone, Somali Region As of 31 January 2020
Ethiopia Access Snapshot - Afar region and Siti zone, Somali region As of 31 January 2020 Afar region is highly prone to natural disasters Afdera The operating environment is highly compromised, with a high such as droughts and seasonal flooding. Long-- risk for humanitarian operations of becoming politicized. In ErebtiDalol Zone 2 term historical grievances coupled with Bidu March 2019, four aid workers were detained by Afar authorities TIGRAY resource-based tensions between ethnic Afar for having allegedly entered the region illegally. They were KunnebaBerahile and its neighbors i.e. Issa (Somali), and Oromo Megale conducting a humanitarian activity in Sitti zone, and decided to Teru Ittu (Amibara woreda) and Karayu (Awash Fentale woreda) in ERITREA overnight in a village of Undufo kebele. In a separate incident, in Yalo AFAR Kurri Red Sea October 2019, an attack by unidentified armed men in Afambo zone 3, and in areas adjacent to Oromia special zone and Amhara- Afdera Robe Town Aso s a Ethnic Somali IDPZone 2016/2018 4 Zone 2 (Kilbet Rasu) Elidar region, continue to cause casualties and forced displacement, Aba 'Ala woreda, Zone 1, near Djibouti, killed a number of civilians spark- Gulina Goba Town limiting partners’ movements and operations. Overall, Ethnican Oromia IDP 2016/2018 L. Afrera Ye'ch'ew ing outrage across the region and prompting peaceful demon- Awra estimated 50,000 people remain displaced, the majority of whom Erebti strations and temporarily road blockages of the Awash highway SNNP Zone 1 Bidu rely almost entirely on assistance provided by host communities. Semera TIGRAYEwa On the other hand, in 2019, the overflow of Awash River and DJIBOUTI Clashes involving Afar and Somali Issa clan continue along Megale Afele Kola flash floods displaced some 3,300 households across six Dubti boundary areas between Afar’s zone 1 and 3 and Sitti zone. -

2019 Myr Oct 25.Pdf (English)
HUMANITARIANHUMANITARIAN RESPONSE PLAN MID-YEAR REVIEW OCTOBER 2019JANUARY - DECEMBER 2019 Photo: ©UNICEF Ethiopia/2019/Nahom Tesfaye ETHIOPIA PEOPLE IN PEOPLE PEOPLE REACHED BY FUNDING STILL # HUMANITARIAN NEED TARGETED 30 JUNE REQUIRED (US$) PARTNERS 8.86M 7.80M 7.93M 319M 58 PART I: THE REVISED Humanitarian RESPONSE PLAN at A GLANCE THE REVISED HUMANITARIAN RESPONSE PLAN AT A GLANCE STRATEGIC OBJECTIVE 1 OPERATIONAL PRESENCE: NUMBER OF PARTNERS Lives are saved and sustained 58 TIGRAY 10 STRATEGIC OBJECTIVE 2 AFAR AMHARA Protection services 12 for affected BENISHANGUL 14 communties are GUMUZ provided 4 DIRE DAWA 7 STRATEGIC OBJECTIVE 3 Addis Ababa HARERI 3 4 Livelihoods and GAMBELA basic services 11 42 delivery are 29 supported to 21 OROMIA strengthen resilience to SOMALI recurrent shocks SNNP 02 PEOPLE IN NEED PEOPLE TARGETED* 8.86M 7.80M REQUIREMENTS (US$) CURRENT GAP (US$) UNTIL THE END OF THE YEAR $1.064B $319M # PEOPLE TARGETED REVISED # PEOPLE TARGETED FUNDING REQUIRED REVISED REQUIREMENTS GOVERNMENT CONTRIBUTIONS INTERNATIONAL GAP SECTOR IN JAN (in millions) IN Oct(in millions) IN JAN (US$ million) IN Sep(US$ million) (US$ million)** CONTRIBUTIONS 2019 (US$ million)** (US$ million) Agricultur 14 08 633 338 102 236 Education 23 21 446 326 83 243 Emer 27 25 1120 800 146 654 Food 80 78 6003 4620 2881 2688 - Health 48 32 1432 951 106 845 44 48 2029 2162 1178 984 Protection 07 11 134 204 79 55 WASH 72 50 1337 1144 223 921 Coor 83 78 52 52 32 20 M Sector not specified - - 76.9 -76.9 Total 1.318B 1.064B 288.1 540.5 319.0 * Inter-sectoral people targeted comes from the food cluster. -

Foods Tabooed for Pregnant Women in Abala District of Afar Region, Ethiopia
Hadush et al. BMC Nutrition (2017) 3:40 DOI 10.1186/s40795-017-0159-x RESEARCH ARTICLE Open Access Foods tabooed for pregnant women in Abala district of Afar region, Ethiopia: an inductive qualitative study Znabu Hadush1*, Zewdie Birhanu2, Mulugeta Chaka2 and Haylay Gebreyesus3 Abstract Background: Food taboo is contributing substantially to malnutrition for pregnant women by restricting and limiting the frequency and variety of foods most of which are nutritious and easily accessible. The practice is common in developing countries and most of the food taboos in East Africa fall on the women and most unfortunately on the pregnant. Foods of animal products, which are the main sources dietary energy of pastoralist communities, are often prone to the practice of food taboos. Nonetheless, the existence of the practice in Ethiopian pastoralist communities, the communities whose way of life is mostly nomadic and based on tending of herds or flocks, is not investigated yet. Therefore, the current study aimed to explore foods tabooed for pregnant women and the reasons behind the practice if exists in Abala district of Afar region, Ethiopia. Methods: Exploratory qualitative study was conducted inductively involving homogeneous participants in four focus group discussions and eight key informants in individual in-depth interview who were purposively selected in Aballa district from March 1 to 30, 2016. A semi-structured interview guide was used to collect the data. The investigators audiotaped focus group discussions and interviews and then transcribed them verbatim. Finally, the transcribed data were imported to Atlas.ti 7 software for coding. Analysis was done inductively. Triangulation and peer debriefing were applied to assure data quality. -

190620 Agriculture Sector Part
ETHIOPIA: AGRICULTURE SECTOR HRP PARTNERS OPERATIONAL PRESENCE - June 2019 TOTAL PARTNERS AND DONORS Partners with Planned, Ongoing and AFAR Completed activities Kunneba 21 Tigray Berahile VSF-G, FAO Aba 'Ala Zone 2 4 15 1 1 15 Central Erebti Bidu NNGO INGO GOV UN DON Gondar Megale Teru Yalo Afar Gonder Elidar Zuria Zone 4 N_Wello Zone 1 Wadla OROMIA Amhara Chifra Asayita Adaa'r CACH, CST, GOAL, SOS Sahel, Telalak Dewe WVI, SCI, DCA, ICRC, SOSVE, AMHARA Beneshangul Dalifage CRS, LWF, MoA, AAH, ERCS LWF, MoA Gumu Zone 5 Haro Limu Dire Chinaksen Tuliguled E_WellegaOromia Dawa Goro Hareri Fafan Muti Fedis Addis Bedeno E_Hararge Midhaga Tola Ababa Babile Daror Jarar (Oromia) Bilcil-bur Gashamo Degehabur Aware Legend Gambela Galhamur Bale Gunagado Shashemene Regional Boundary Zuria Doolo West Goro (Bale) Zone Boundary Arsi Rayitu Somali Abaya Dawe Bore Bule Girja Ketchen Afder Gedeo (Harenfema) Shabelle Partners at Woreda Level SNNPR Uraga Elkare/Serer SOMALI Kercha Guji Aga Wayu Kelafo IRE, VSF-S, ICRC, South West Charati/Weyib Hargele Adadle Mustahil 1 Guji Liben Ferfer ERCS, FAO, OXFAM Omo Deka Gumi suftu Liban 2 Yabelo Arero Idalo Dasenech Borena SNNPR Barey (Kuraz) Wachile Dubluk Hudet CST, WVI, SCI Dolo Odo 3 Dilo Dhas Daawa Dire Moyale Moyale MCMDO, MoA (Oromia) Government Miyo (Somali) Creation date: 20th June 2019 Sources: Response target figures and funding data were colleceted and acompiled from the information submitted by Agriculture Sector partners as of 30 May 2019. Feedback: Espico Iga (Denis) & Hudad Ibrahima, Information Management -

LIFE AMIDST a PANDEMIC: Hunger, Migration and Displacement in the East and Horn of Africa
LIFE AMIDST A PANDEMIC: Hunger, Migration and Displacement in the East and Horn of Africa June 2021 ACKNOWLEDGEMENTS This report is the first joint publication of its kind by the World Food Programme (WFP) and the International Organization for Migration (IOM) for the East and Horn of Africa region. Facilitated by WFP’s Research, Assessment and Monitoring Division and IOM’s Regional Data Hub for the East and Horn of Africa, the report reflects multiple inputs from both organizations. Both agencies would like to express gratitude and appreciation to the authors of the report including Marianne Jensby, Andrea Breslin, Dion McDougal, Kennedy Nanga, Zaccheus Ndirima and Krishna Pahari (WFP); and Naomi Burnett, Zineb Homman Loudiye, Chiara Lucchini Gilera, Amalraj Nallainathan, Laura Nistri and Asma Saeed (IOM). This report would not have been possible without the participation and contributions of colleagues in the field. The authors would particularly like to thank country and regional offices’ colleagues from both organizations for their support and expertise, and the data and analysis provided for the case studies featured in this publication. Contributions by WFP’s Regional Emergency Preparedness and Response unit and IOM’s Departments of Operations and Emergencies, of Migration Management and the Displacement Tracking Matrix programme were also invaluable. Special thanks is extended to all those involved in the editing, design, proofreading of the report, particularly Pauline Muchigi (WFP) and Zeina Wairoa (IOM). LIFE AMIDST A PANDEMIC: -

WFP Ethiopia Country Office THIRD PARTY MONITORING SERVICES Call for Proposals Ref No: CFP/TPM/CSP/2020/002 Closing Date and Time: 30 June 2021 at 23:59
WFP Ethiopia Country Office THIRD PARTY MONITORING SERVICES Call for Proposals Ref No: CFP/TPM/CSP/2020/002 Closing Date and Time: 30 June 2021 at 23:59 Background Under the nutrition response, WFP supports the full implementation of integrated management of acute malnutrition (IMAM) interventions, working with the Ministry of Health (MoH), United Nations Children’s Fund (UNICEF) and NGOs to increase the number of woredas with IMAM in the Oromia, Afar, SNNP and Sidama regions. Where IMAM has been incorporated into the government health system, targeted supplementary feeding supports routine monthly nutrition screening to identify children under five and pregnant and lactating women and girls (PLWG) with moderate acute malnutrition and enrol them for treatment, complemented by community mobilization, audience-targeted SBCC and capacity strengthening of the MoH and its partners. WFP is committed to ensuring that its ability to collect and compile an evidence base that informs programming is not compromised. To carry out an effective and timely assessment, delivery, distribution and monitoring of food assistance, and to ensure the safety of its personnel, WFP Ethiopia outsources its monitoring activities to overcome monitoring access constraints as well as to improve efficiency due to the need for a large number of monitors beyond WFP’s current regular staffing capacity. Low monitoring coverage is an institutional and programmatic risk that compromises the ability of WFP to collect evidence-based data needed to inform operations. Under the current system in the Oromia, Afar, SNNP and Sidama regions, food is transported to distribution sites by commercial transporters organized by Regional Disaster Risk Management Bureaus and Regional Health Bureau.