Synthesis of Alkenes: Claisen Rearrangement of Allyl Vinyl Ethers, Part Iii; Mechanistic Views; the Organic Chemistry Notebook Series, a Didactical Approach, Nº 11
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Study of the Thermal Stability of Benzyl 2,4-Dimethyl-6
A STUDY OF THE THERMAL STABILITY OF BENZYL 2 1 4·DIMETHYL•6•PROPENYLPEENYL ETHER by RONALD JENE DUPZIK A THESIS submitted to OREGON STATE COLLEGE in partial fulfillment ot the requirements tor the degree ot MASTER OF SCIENCE June 1958 APPEOYED I Redacted for Privacy tarogl,rtr Frofrlter of 0rnt In 0hrrgr of, I{aJor Redacted for Privacy Chrlrnen of Drprntmat of Shontrtrf Redacted for Privacy Ghelrura of Bahool &adurtc 0omlttm Redacted for Privacy Deur of 0mdu*tr $rhoel Drtc thrrtr lr prrrontrd Ausust 7, 1957 Sypcd by l{*y ldrnr 'l'o M;y Mothez in memory ot My Father ACKNOWLEDGMENT The author wishes to expreaa hia apprecia tion to Dr. Elliot N. Marvell whose help and guidance has made this work possible. Thanks are given to Dr. B. E. Christensen and Dr . c. s. Pease who have so graciously helped in Dr. Marvell's absence. To Miss Isabelle Forbusoh for her patience and help in the preparation of this manuscript. And, to the Martinez Research Laboratory ot the Shell Oil Company for the infrared and ultraviolet spectral analysis of the new compounds. TABLE OF CONTENTS Pa!e INTRODUCTION • • • • • • • • • • • • • • • • • • 1 HISTORICAL • • • • • • • • • • • . •. • • • • • • 3 DISCUSSION • • • • • • • • • • • • • • • • • • • • 16 EXPERIMENTAL • • • • • • • • • • • • • • • • • • 29 Synthesis A • • • • • • 29 Synthesis B • • • • • • • • • • • • • • • 31 Rearrangement and Thermal Stability • • • • • 37 SUMMARY. • • • • • • • • • • • • • 40 BI BLIOGRAPHY • • • • • • • • • • • • • • • • • • • 41 LIST OF FIGURES Figure 1 • • • " • • • • • • • • • • -

Allicin in Blood.Pdf
European Journal of Medicinal Chemistry 45 (2010) 1912–1918 Contents lists available at ScienceDirect European Journal of Medicinal Chemistry journal homepage: http://www.elsevier.com/locate/ejmech Original article Reaction mechanisms of allicin and allyl-mixed disulfides with proteins and small thiol molecules Talia Miron a,*, Irving Listowsky b, Meir Wilchek a a Department of Biological Chemistry, Weizmann Institute of Science, Rehovot 76100, Israel b Department of Biochemistry, Albert-Einstein College of Medicine, Bronx, NY, USA article info abstract Article history: Allylsulfides from garlic are chemopreventive agents. Entering cells they are expected to initially interact Received 15 October 2009 with glutathione. Accordingly, reaction mechanisms of the product, S-allylthio-glutathione, with model Received in revised form proteins and thiols were analyzed in cell free systems. With glutathionyl, cysteinyl or captopril repre- 14 January 2010 senting S-allyl aliphatic adducts, the reaction with sulfhydryl groups resulted in mixed disulfide Accepted 15 January 2010 mixtures, formed by both, S-allyl and aliphatic moieties. Available online 21 January 2010 To improve conventional prodrug treatment of blood pressure, cancer and intestinal inflammation S-allylthio prodrugs, such as S-allylthio-6-mercaptopurine and S-allylthio-captopril were synthesized. Keywords: Allicin Synergistic activities of the 2 constituents, as well as increased cell permeability allow for efficient in vivo Glutathione activity. Upon reaction of these derivatives with glutathione, S-allylthio-glutathione is formed, while S-Allylthio-mixed disulfide 6-mercaptopurine is the leaving group. Excess cellular glutathione enables several cycles of sulfhydryl- Prodrug disulfide exchange reactions to occur, extending the hybrid drug’s pharmacodynamics. Mechanism of action Ó 2010 Elsevier Masson SAS. -

Synthesis of Y,Δ-Unsaturated Amino Acids by Claisen Rearrangement - Last 25 Years
The Free Internet Journal Review for Organic Chemistry Archive for Arkivoc 2021, part ii, 0-0 Organic Chemistry to be inserted by editorial office Synthesis of y,δ-unsaturated amino acids by Claisen rearrangement - last 25 years Monika Bilska-Markowska,a Marcin Kaźmierczak,*a,b and Henryk Koroniaka aFaculty of Chemistry, Adam Mickiewicz University in Poznań, Uniwersytetu Poznańskiego 8, 61-614 Poznań, Poland bCentre for Advanced Technologies, Adam Mickiewicz University in Poznań, Uniwersytetu Poznańskiego 10, 61-614 Poznań, Poland Email: [email protected] In dedication to Professor Zbigniew Czarnocki on the occasion of his 66th anniversary Received mm-dd-yyyy Accepted mm-dd-yyyy Published on line mm-dd-yyyy Dates to be inserted by editorial office Abstract This mini review summarizes achievements in the synthesis of y,δ-unsaturated amino acids via Claisen rearrangements. The multitude of products that can be obtained using the discussed protocol shows that it is one of the most important reactions in organic synthesis. Moreover, many Claisen rearrangement products are building blocks in the synthesis of more complex molecules with potential biological activity. Keywords: y,δ-Unsaturated amino acids, Claisen rearrangement, fluorine-containing γ,δ-unsaturated amino acids, diastereoselectivity, optically active compounds DOI: https://doi.org/10.24820/ark.5550190.p011.335 Page 1 ©AUTHOR(S) Arkivoc 2021, ii, 0-0 Bilska-Markowska, M. et al. Table of Contents 1. Introduction 2. Chelated Claisen Rearrangement 3. Related Versions of Claisen Rearrangement for γ,δ-Unsaturated Amino Acids 4. Application of Claisen Rearrangement to the Synthesis of Fluorine-containing γ,δ-Unsaturated Amino Acids 5. -

Proton Nmr Spectroscopy
1H NMR Spectroscopy (#1c) The technique of 1H NMR spectroscopy is central to organic chemistry and other fields involving analysis of organic chemicals, such as forensics and environmental science. It is based on the same principle as magnetic resonance imaging (MRI). This laboratory exercise reviews the principles of interpreting 1H NMR spectra that you should be learning right now in Chemistry 302. There are four questions you should ask when you are trying to interpret an NMR spectrum. Each of these will be discussed in detail. The Four Questions to Ask While Interpreting Spectra 1. How many different environments are there? The number of peaks or resonances (signals) in the spectrum indicates the number of nonequivalent protons in a molecule. Chemically equivalent protons (magnetically equivalent protons) give the same signal in the NMR whereas nonequivalent protons give different signals. 1 For example, the compounds CH3CH3 and BrCH2CH2Br all have one peak in their H NMR spectra because all of the protons in each molecule are equivalent. The compound below, 1,2- dibromo-2-methylpropane, has two peaks: one at 1.87 ppm (the equivalent CH3’s) and the other at 3.86 ppm (the CH2). 1.87 1.87 ppm CH 3 3.86 ppm Br Br CH3 1.87 ppm 3.86 10 9 8 7 6 5 4 3 2 1 0 2. How many 1H are in each environment? The relative intensities of the signals indicate the numbers of protons that are responsible for individual signals. The area under each peak is measured in the form of an integral line. -

Mechanism and Selectivity of Rhodium-Catalyzed 1:2 Coupling Of
Article pubs.acs.org/JACS Terms of Use Mechanism and Selectivity of Rhodium-Catalyzed 1:2 Coupling of Aldehydes and Allenes Genping Huang, Marcin Kalek, and Fahmi Himo* Department of Organic Chemistry, Arrhenius Laboratory, Stockholm University, SE-106 91 Stockholm, Sweden *S Supporting Information ABSTRACT: The rhodium-catalyzed highly regioselective 1:2 coupling of aldehydes and allenes was investigated by means of density functional theory calculations. Full free energy profiles were calculated, and several possible reaction pathways were evaluated. It is shown that the energetically most plausible catalytic cycle is initiated by oxidative coupling of the two allenes, which was found to be the rate-determining step of the overall reaction. Importantly, Rh−allyl complexes that are able to adopt both η3 and η1 configurations were identified as key intermediates present throughout the catalytic cycle with profound implications for the selectivity of the reaction. The calculations reproduced and rationalized the experimentally observed selectivities and provided an explanation for the remarkable alteration in the product distribution when the catalyst precursor is changed from [RhCl(nbd)]2 (nbd = norbornadiene) to complexes containing noncoordinating counterions ([Rh(cod)2X]; X = OTf, BF4,PF6; cod = 1,5-cyclooctadiene). It turns out that the overall selectivity of the reaction is controlled by a combination of the inherent selectivities of several of the elementary steps and that both the mechanism and the nature of the selectivity-determining steps change when the catalyst is changed. 1. INTRODUCTION comes from the laboratory of Murakami and consists of a 1:2 6 Rhodium(I) complexes are well-established catalysts for three- coupling of aldehydes and allenes (Scheme 2b). -

Ring Opening of Donor–Acceptor Cyclopropanes with N-Nucleo- Philes
SYNTHESIS0039-78811437-210X © Georg Thieme Verlag Stuttgart · New York 2017, 49, 3035–3068 short review 3035 en Syn thesis E. M. Budynina et al. Short Review Ring Opening of Donor–Acceptor Cyclopropanes with N-Nucleo- philes Ekaterina M. Budynina* Konstantin L. Ivanov Ivan D. Sorokin Mikhail Ya. Melnikov Lomonosov Moscow State University, Department of Chemistry, Leninskie gory 1-3, Moscow 119991, Russian Federation [email protected] Received: 06.02.2017 Accepted after revision: 07.04.2017 Published online: 18.05.2017 DOI: 10.1055/s-0036-1589021; Art ID: ss-2017-z0077-sr Abstract Ring opening of donor–acceptor cyclopropanes with various N-nucleophiles provides a simple approach to 1,3-functionalized com- pounds that are useful building blocks in organic synthesis, especially in assembling various N-heterocycles, including natural products. In this review, ring-opening reactions of donor–acceptor cyclopropanes with amines, amides, hydrazines, N-heterocycles, nitriles, and the azide ion are summarized. 1 Introduction 2 Ring Opening with Amines Ekaterina M. Budynina studied chemistry at Lomonosov Moscow 3 Ring Opening with Amines Accompanied by Secondary Processes State University (MSU) and received her Diploma in 2001 and Ph.D. in Involving the N-Center 2003. Since 2013, she has been a leading research scientist at Depart- 3.1 Reactions of Cyclopropane-1,1-diesters with Primary and Secondary ment of Chemistry MSU, focusing on the reactivity of activated cyclo- Amines propanes towards various nucleophilic agents, as well as in reactions -

Catalyzed Claisen Rearrangement of Allenyl Vinyl Ethers: a Synthetic and Mechanistic Approach Kassem M
Florida State University Libraries Electronic Theses, Treatises and Dissertations The Graduate School 2011 Gold (I)-Catalyzed Claisen Rearrangement of Allenyl Vinyl Ethers: A Synthetic and Mechanistic Approach Kassem M. Hallal Follow this and additional works at the FSU Digital Library. For more information, please contact [email protected] THE FLORIDA STATE UNIVERSITY COLLEGE OF ARTS AND SCIENCES GOLD (I)-CATALYZED CLAISEN REARRANGEMENT OF ALLENYL VINYL ETHERS; A SYNTHETIC AND MECHANISTIC APPROACH By KASSEM M. HALLAL A Dissertation submitted to the Department of Chemistry and Biochemistry in partial fulfillment of the requirements for the degree of Doctor of Philosophy Degree Awarded: Spring Semester, 2011 The members of the committee approve the dissertation of Kassem M. Hallal defended on March 18, 2011. _______________________________________ Marie E. Krafft Professor Directing Dissertation _______________________________________ Thomas C. S. Keller III University Representative _______________________________________ Robert A. Holton Committee Member _______________________________________ Gregory B. Dudley Committee Member _______________________________________ William T. Cooper Committee Member Approved: ____________________________________________________________ Joseph B. Schlenoff, Chair, Department of Chemistry and Biochemistry The Graduate School has verified and approved the above-named committee members. ii This work is dedicated To My soul mate, lovely wife zeinab, My baby Mohammad And also to my parents Mohammad & Kamela And to My brothers and lovely sister Youssef, Hamzeh and Fatima & My Great professor Prof. Marie E. Krafft iii ACKNOWLEDGEMENTS Starting my PhD career at Florida State University was one of the most important stages in my life. Throughout the past five years, I learned a lot of things about chemistry and science, however, the most important thing which I learned was the chemistry of life. -
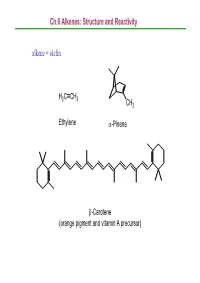
Ch.6 Alkenes: Structure and Reactivity Alkene = Olefin
Ch.6 Alkenes: Structure and Reactivity alkene = olefin H2CCH2 CH3 Ethylene α-Pinene β-Carotene (orange pigment and vitamin A precursor) Ch.6 Alkenes: Structure and Reactivity 6.1 Industrial Preparation and Use of Alkenes Compounds derived industrially from ethylene CH3CH2OH Ethanol CH3CHO Acetaldehyde CH3COOH Acetic acid HOCH2CH2OH Ethylene glycol ClCH2CH2Cl Ethylene dichloride H C=CHCl Vinyl chloride H2CCH2 2 O Ethylene oxide Ethylene (26 million tons / yr) O Vinyl acetate O Polyethylene Ch.6 Alkenes: Structure and Reactivity Compounds derived industrially from propylene OH Isopropyl alcohol H3CCH3 O Propylene oxide CH3 H3CCH CH2 Propylene Cumene (14 million tons / yr) CH3 CH3 Polypropylene Ch.6 Alkenes: Structure and Reactivity • Ethylene, propylene, and butene are synthesized industrially by thermal cracking of natural gas (C1-C4 alkanes) and straight-run gasoline (C4-C8 alkanes). 850-900oC CH (CH ) CH H + CH + H C=CH + CH CH=CH 3 2 n 3 steam 2 4 2 2 3 2 + CH3CH2CH=CH2 - the exact processes are complex; involve radical process H 900oC CH3CH2 CH2CH3 22H2CCH H2C=CH2 +H2 Ch.6 Alkenes: Structure and Reactivity • Thermal cracking is an example of a reaction whose energetics are dominated by entropy (∆So) rather than enthalpy (∆Ho) in the free-energy equation (∆Go = ∆Ho -T∆So) . ; C-C bond cleavage (positive ∆Ho) ; high T and increased number of molecules → larger T∆So Ch.6 Alkenes: Structure and Reactivity 6.2 Calculating Degree of Unsaturation unsaturated: formula of alkene CnH2n ; formula of alkane CnH2n+2 in general, each ring or double -

A New Reaction Mechanism of Claisen Rearrangement Induced by Few-Optical-Cycle Pulses: Demonstration of Nonthermal Chemistry by Femtosecond Vibrational Spectroscopy*
Pure Appl. Chem., Vol. 85, No. 10, pp. 1991–2004, 2013. http://dx.doi.org/10.1351/PAC-CON-12-12-01 © 2013 IUPAC, Publication date (Web): 13 August 2013 A new reaction mechanism of Claisen rearrangement induced by few-optical-cycle pulses: Demonstration of nonthermal chemistry by femtosecond vibrational spectroscopy* Izumi Iwakura1,2,3,‡, Atsushi Yabushita4, Jun Liu2, Kotaro Okamura2, Satoko Kezuka5, and Takayoshi Kobayashi2 1Innovative Use of Light and Materials/Life, PRESTO, JST, 4-1-8 Honcho, Kawaguchi, Saitama, 332-0012, Japan; 2University of Electro-Communications, 1-5-1 Chofugaoka, Chofu, Tokyo 182-8585, Japan; 3Faculty of Engineering, Kanagawa University, 3-27-1 Rokkakubashi, Yokohama 221-8686, Japan; 4Department of Electrophysics, National Chiao-Tung University, Hsinchu 300, Taiwan; 5Department of Applied Chemistry, School of Engineering, Tokai University, 1117 Kitakaname, Hiratsuka-shi, Kanagawa 259-1292, Japan Abstract: Time-resolved vibration spectroscopy is the only known way to directly observe reaction processes. In this work, we measure time-resolved vibration spectra of the Claisen rearrangement triggered and observed by few-optical-cycle pulses. Changes in molecular structure during the reaction, including its transition states (TSs), are elucidated by observ- ing the transient changes of molecular vibration wavenumbers. We pump samples with visi- ble ultrashort pulses of shorter duration than the molecular vibration period, and with photon energies much lower than the minimum excitation energy of the sample. The results indicate that the “nonthermal Claisen rearrangement” can be triggered by visible few-optical-cycle pulses exciting molecular vibrations in the electronic ground state of the sample, which replaces the typical thermal Claisen rearrangement. -

Ganic Compounds
6-1 SECTION 6 NOMENCLATURE AND STRUCTURE OF ORGANIC COMPOUNDS Many organic compounds have common names which have arisen historically, or have been given to them when the compound has been isolated from a natural product or first synthesised. As there are so many organic compounds chemists have developed rules for naming a compound systematically, so that it structure can be deduced from its name. This section introduces this systematic nomenclature, and the ways the structure of organic compounds can be depicted more simply than by full Lewis structures. The language is based on Latin, Greek and German in addition to English, so a classical education is beneficial for chemists! Greek and Latin prefixes play an important role in nomenclature: Greek Latin ½ hemi semi 1 mono uni 1½ sesqui 2 di bi 3 tri ter 4 tetra quadri 5 penta quinque 6 hexa sexi 7 hepta septi 8 octa octo 9 ennea nona 10 deca deci Organic compounds: Compounds containing the element carbon [e.g. methane, butanol]. (CO, CO2 and carbonates are classified as inorganic.) See page 1-4. Special characteristics of many organic compounds are chains or rings of carbon atoms bonded together, which provides the basis for naming, and the presence of many carbon- hydrogen bonds. The valency of carbon in organic compounds is 4. Hydrocarbons: Compounds containing only the elements C and H. Straight chain hydrocarbons are named according to the number of carbon atoms: CH4, methane; C2H6 or H3C-CH3, ethane; C3H8 or H3C-CH2-CH3, propane; C4H10 or H3C-CH2- CH2-CH3, butane; C5H12 or CH3CH2CH2CH2CH3, pentane; C6H14 or CH3(CH2)4CH3, hexane; C7H16, heptane; C8H18, octane; C9H20, nonane; C10H22, CH3(CH2)8CH3, decane. -

Total Synthesis of Malformin C, an Inhibitor of Bleomycin- Induced G2
J. Antibiot. 61(5): 297–302, 2008 THE JOURNAL OF ORIGINAL ARTICLE ANTIBIOTICS Total Synthesis of Malformin C, an Inhibitor of Bleomycin- Induced G2 Arrest Yasuhiro Kojima, Toshiaki Sunazuka, Kenichiro Nagai, Khachatur Julfakyan, Takashi Fukuda, Hiroshi Tomoda, Satoshi O¯ mura Received: April 14, 2008 / Accepted: May 3, 2008 © Japan Antibiotics Research Association Abstract Total synthesis of a fungal cyclic peptide, Jurkat cells with IC50 values of 480 nM and 0.9 nM, malformin C, recently rediscovered as a G2 checkpoint respectively [2]. Malformin A1 is a bicyclic pentapeptide inhibitor was completed. Our synthesis involved a containing one L-isoleucine, one D-leucine, one L-valine, convergent approach with respect to a linear pentapeptide, and two D-cysteines, while malformin C has L-leucine cyclization, and oxidative disulfide formation. instead of L-isoleucine. Interestingly, this slight structural difference of the side-chains has an influence on the Keywords total synthesis, malformin C, G2 checkpoint inhibitory activity. Consequently, malformin C is a inhibitor, anti-cancer reagent, cyclic peptide, disulfide promising candidate as a potentiator of anti-cancer agents. Although malformins are also known to show a variety of activities such as inducing root curvatures and Introduction malformations in plants, antibacterial activity and enhanced fibrinolytic activity [3ϳ6], the interesting bioactivity of Spontaneous and chemical damage to DNA induces signal malformin C, as well as its bicyclic structure with a transduction pathways called checkpoints, which delay cell disulfide-bond bridge, prompted us to study the synthesis cycle progression and repair of DNA [1]. DNA damage in of this class of cyclic peptides. Herein, we describe a normal human cells can be repaired in both the G1 and G2 synthesis of malformin C. -

Surface Chemistry Changes of Weathered HDPE/Wood-Flour
Polymer Degradation and Stability 86 (2004) 1–9 www.elsevier.com/locate/polydegstab Surface chemistry changes of weathered HDPE/wood-flour composites studied by XPS and FTIR spectroscopy* Nicole M. Starka,), Laurent M. Matuanab aU.S. Department of Agriculture, Forest Service, Forest Products Laboratory, One Gifford Pinchot Drive, Madison, WI 53726-2398, United States bDepartment of Forestry, Michigan State University, East Lansing, MI 48824-1222, United States Received 7 August 2003; received in revised form 3 November 2003; accepted 4 November 2003 Abstract The use of wood-derived fillers by the thermoplastic industry has been growing, fueled in part by the use of wood-fiber– thermoplastic composites by the construction industry. As a result, the durability of wood-fiber–thermoplastic composites after ultraviolet exposure has become a concern. Samples of 100% high-density polyethylene (HDPE) and HDPE filled with 50% wood- flour (WF) were weathered in a xenon arc-type accelerated weathering apparatus for 2000 h. Changes in surface chemistry were studied using spectroscopic techniques. X-ray photoelectron spectroscopy (XPS) was used to verify the occurrence of surface oxidation. Fourier transform infrared (FTIR) spectroscopy was used to monitor the development of degradation products, such as carbonyl groups and vinyl groups, and to determine changes in HDPE crystallinity. The results indicate that surface oxidation occurred immediately after exposure for both the neat HDPE and WF/HDPE composites; the surface of the WF/HDPE composites was oxidized to a greater extent than that of the neat HDPE. This suggests that the addition of WF to the HDPE matrix results in more weather-related damage.