Potassium Currents During the Action Potential of Hippocampal CA3 Neurons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Long QT Syndrome: from Channels to Cardiac Arrhythmias
Long QT syndrome: from channels to cardiac arrhythmias Arthur J. Moss, Robert S. Kass J Clin Invest. 2005;115(8):2018-2024. https://doi.org/10.1172/JCI25537. Review Series Long QT syndrome, a rare genetic disorder associated with life-threatening arrhythmias, has provided a wealth of information about fundamental mechanisms underlying human cardiac electrophysiology that has come about because of truly collaborative interactions between clinical and basic scientists. Our understanding of the mechanisms that control the critical plateau and repolarization phases of the human ventricular action potential has been raised to new levels through these studies, which have clarified the manner in which both potassium and sodium channels regulate this critical period of electrical activity. Find the latest version: https://jci.me/25537/pdf Review series Long QT syndrome: from channels to cardiac arrhythmias Arthur J. Moss1 and Robert S. Kass2 1Heart Research Follow-up Program, Department of Medicine, University of Rochester School of Medicine and Dentistry, Rochester, New York, USA. 2Department of Pharmacology, Columbia University Medical Center, New York, New York, USA. Long QT syndrome, a rare genetic disorder associated with life-threatening arrhythmias, has provided a wealth of information about fundamental mechanisms underlying human cardiac electrophysiology that has come about because of truly collaborative interactions between clinical and basic scientists. Our understanding of the mecha- nisms that control the critical plateau and repolarization phases of the human ventricular action potential has been raised to new levels through these studies, which have clarified the manner in which both potassium and sodium channels regulate this critical period of electrical activity. -

7.016 Introductory Biology Fall 2018
7.016: Fall 2018: MIT 7.016 Recitation 15 – Fall 2018 (Note: The recitation summary should NOT be regarded as the substitute for lectures) (This material is COPYRIGHT protected.) Summary of Lecture 22 (11/2): Neurons and action potentials: Ions can move across membranes through pumps and channels. Integral membrane proteins like these cross the membrane via transmembrane domains. Pumps are ATPases that set up the concentration gradients of ions across cell membranes, such that K+ is high inside cells and other ions (such as Cl–, Na+, and Ca2+) are high outside cells. A membrane potential is only set up by ions that move freely across the membrane through open channels, creating one side of the membrane that is more positive relative to the other side. This movement of ions does not dissipate the concentration gradient because the number of ions that move to generate a membrane potential is very small compared to the number of ions that need to be pumped to create a concentration gradient. Most cell membranes only contain open K+ channels and thus only K+ flows freely across membranes through channels; K+ is high inside, causing it to flow outside, giving the inside of cells a negative membrane potential. Ions can move across the membrane through open ion channels. Two forces act to dictate this movement – the concentration gradient and the electrical gradient. Ions move down their concentration gradient through channels, and ions move towards the side of the membrane that harbors the opposite charge. Neurons are the cells of your nervous system that make connections with each other to transmit impulse/ signals. -

Neurotransmitter Actions
Central University of South Bihar Panchanpur, Gaya, India E-Learning Resources Department of Biotechnology NB: These materials are taken/borrowed/modified/compiled from various resources like research articles and freely available internet websites, and are meant to be used solely for the teaching purpose in a public university, and for serving the needs of specified educational programmes. Dr. Jawaid Ahsan Assistant Professor Department of Biotechnology Central University of South Bihar (CUSB) Course Code: MSBTN2003E04 Course Name: Neuroscience Neurotransmitter Actions • Excitatory Action: – A neurotransmitter that puts a neuron closer to an action potential (facilitation) or causes an action potential • Inhibitory Action: – A neurotransmitter that moves a neuron further away from an action potential • Response of neuron: – Responds according to the sum of all the neurotransmitters received at one time Neurotransmitters • Acetylcholine • Monoamines – modified amino acids • Amino acids • Neuropeptides- short chains of amino acids • Depression: – Caused by the imbalances of neurotransmitters • Many drugs imitate neurotransmitters – Ex: Prozac, zoloft, alcohol, drugs, tobacco Release of Neurotransmitters • When an action potential reaches the end of an axon, Ca+ channels in the neuron open • Causes Ca+ to rush in – Cause the synaptic vesicles to fuse with the cell membrane – Release the neurotransmitters into the synaptic cleft • After binding, neurotransmitters will either: – Be destroyed in the synaptic cleft OR – Taken back in to surrounding neurons (reuptake) Excitable cells: Definition: Refers to the ability of some cells to be electrically excited resulting in the generation of action potentials. Neurons, muscle cells (skeletal, cardiac, and smooth), and some endocrine cells (e.g., insulin- releasing pancreatic β cells) are excitable cells. -

Genetic Alteration of the Metal/Redox Modulation of Cav3.2 T-Type
Genetic alteration of the metal/redox modulation of Cav3.2 T-type calcium channel reveals its role in neuronal excitability Tiphaine Voisin, Emmanuel Bourinet, Philippe Lory To cite this version: Tiphaine Voisin, Emmanuel Bourinet, Philippe Lory. Genetic alteration of the metal/redox mod- ulation of Cav3.2 T-type calcium channel reveals its role in neuronal excitability. The Journal of Physiology, Wiley, 2016, 594 (13), pp.3561–74. 10.1113/JP271925. hal-01940961 HAL Id: hal-01940961 https://hal.archives-ouvertes.fr/hal-01940961 Submitted on 30 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. J Physiol 594.13 (2016) pp 3561–3574 3561 Genetic alteration of the metal/redox modulation of Cav3.2 T-type calcium channel reveals its role in neuronal excitability Tiphaine Voisin1,2,3, Emmanuel Bourinet1,2,3 and Philippe Lory1,2,3 1Centre National pour la Recherche Scientifique UMR 5203, D´epartement de Physiologie, Institut de G´enomique Fonctionnelle, Universit´edeMontpellier, Montpellier, F-34094 France 2Institut National de la Sant´eetdelaRechercheM´edicale, U 1191, Montpellier, F-34094 France Neuroscience 3LabEx ‘Ion Channel Science and Therapeutics’, Montpellier, F-34094 France Key points r In this study, we describe a new knock-in (KI) mouse model that allows the study of the H191-dependent regulation of T-type Cav3.2 channels. -

Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M
Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M. Baumgarten, Ph.D. OBJECTIVES: 1. Describe the basic characteristics of cardiac electrical activity and the spread of the action potential through the heart 2. Compare the characteristics of action potentials in different parts of the heart 3. Describe how serum K modulates resting potential 4. Describe the ionic basis for the cardiac action potential and changes in ion currents during each phase of the action potential 5. Identify differences in electrical activity across the tissues of the heart 6. Describe the basis for normal automaticity 7. Describe the basis for excitability 8. Describe the basis for conduction of the cardiac action potential 9. Describe how the responsiveness relationship and the Na+ channel cycle modulate cardiac electrical activity I. BASIC ELECTROPHYSIOLOGIC CHARACTERISTICS OF CARDIAC MUSCLE A. Electrical activity is myogenic, i.e., it originates in the heart. The heart is an electrical syncitium (i.e., behaves as if one cell). The action potential spreads from cell-to-cell initiating contraction. Cardiac electrical activity is modulated by the autonomic nervous system. B. Cardiac cells are electrically coupled by low resistance conducting pathways gap junctions located at the intercalated disc, at the ends of cells, and at nexus, points of side-to-side contact. The low resistance pathways (wide channels) are formed by connexins. Connexins permit the flow of current and the spread of the action potential from cell-to-cell. C. Action potentials are much longer in duration in cardiac muscle (up to 400 msec) than in nerve or skeletal muscle (~5 msec). -
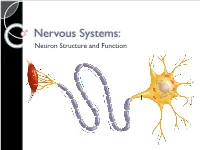
4-Nervous-System-Structure-PPT.Pdf
Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation and coordination that produce coherency and result in harmonious function. Integration Cellular integration = processes within cells Whole-animal integration = selective combination and processing of sensory, endocrine, and central nervous system (CNS) information in ways that promote harmonious functioning of the whole organism within its environment. ◦ This includes its all its cells, tissues, and organs Integration Nerve cells are specialized for control and coordination. Integration ensures that an animal’s responses are smooth and coordinated rather than clashing or disjointed. Excitable Cells Neurons are a type of excitable cell ◦ Specially adapted to generate an electrical signal Can rapidly alter their membrane potential in response to an incoming signal. Vary in structure and function but use the same basic mechanism to send signals. Neuron Function – Main Points Specialized for processing and conveying information. Information is coded into changes in electrical potential across cell membranes. Neurons use action potentials to transmit these signals across long distances. Neurons allow animals to sense and respond to their environment. Benefits of Neurons Plants (no neurons): Action potentials travel @ 1-3 cm/sec Animals (neurons): Action potentials travel @ 100m/sec or 10,000cm/sec CNS to Muscles Signal Reception Dendrites & Cell Body Signal Integration Axon Hillock Signal Conduction Axon Signal Transmission Axon Terminals Signal Reception Dendrites sense and convert incoming signals into electrical signals by changing membrane potential. Cell Body = routine metabolic functions Signal Integration Incoming signals are conducted to the axon hillock If signal is sufficiently large an electrical signal, or action potential, is initiated. -

Electrolyte Disorders and Arrhythmogenesis
Cardiology Journal 2011, Vol. 18, No. 3, pp. 233–245 Copyright © 2011 Via Medica REVIEW ARTICLE ISSN 1897–5593 Electrolyte disorders and arrhythmogenesis Nabil El-Sherif1, Gioia Turitto2 1State University of NY, Downstate Medical Center and NY Harbor VA Healthcare System, Brooklyn, NY, USA 2Methodist University Hospital, Brooklyn, NY, USA Abstract Electrolyte disorders can alter cardiac ionic currents kinetics and depending on the changes can promote proarrhythmic or antiarrhythmic effects. The present report reviews the mecha- nisms, electrophysiolgical (EP), electrocardiographic (ECG), and clinical consequences of elec- trolyte disorders. Potassium (K+) is the most abundent intracellular cation and hypokalemia is the most commont electrolyte abnormality encountered in clinical practice. The most signifcant ECG manifestation of hypokalemia is a prominent U wave. Several cardiac and + non cardiac drugs are known to suppress the HERG K channel and hence the IK, and especially in the presence of hypokalemia, can result in prolonged action potential duration and QT interval, QTU alternans, early afterdepolarizations, and torsade de pointes ventricu- lar tachyarrythmia (TdP VT). Hyperkalemia affects up to 8% of hospitalized patients mainly in the setting of compromised renal function. The ECG manifestation of hyperkalemia de- pends on serum K+ level. At 5.5–7.0 mmol/L K+, tall peaked, narrow-based T waves are seen. At > 10.0 mmol/L K+, sinus arrest, marked intraventricular conduction delay, ventricular techycardia, and ventricular fibrillation can develop. Isolated abnormalities of extracellular calcium (Ca++) produce clinically significant EP effects only when they are extreme in either direction. Hypocalcemia, frequently seen in the setting of chronic renal insufficiency, results in prolonged ST segment and QT interval while hypercalcemia, usually seen with hyperparathy- roidism, results in shortening of both intervals. -

Two Components of the Cardiac Action Potential I
Two Components of the Cardiac Action Potential I. Voltage-time course and the effect of acet.flcholine on atrial and nodal cells of the rabbit heart ANTONIO PAES de CARVALHO, BRIAN FRANCIS HOFFMAN, and MARILENE de PAULA CARVALHO From the Instituto de Biofisica da Universidade Federal do Rio de Janeiro, Rio de Janeiro (GB), Brazil, and the Department of Pharmacology, College of Physicians and Surgeons, Columbia University, New York 10032 ABSTRACT Transmembrane potentials recorded from the rabbit heart in vitro were displayed as voltage against time (V, t display), and dV/dt against voltage (I?, V or phase-plane display). Acetylcholine was applied to the record- ing site by means of a hydraulic system. Results showed that (a) differences in time course of action potential upstroke can be explained in terms of the rela- tive magnitude of fast and slow phases of depolarization; (b) acetylcholine is capable of depressing the slow phase of depolarization as well as the plateau of the action potential; and (¢) action potentials from nodal (SA and AV) cells seem to lack the initial fast phase. These results were construed to support a two-component hypothesis for cardiac electrogenesis. The hypothesis states that cardiac action potentials are composed of two distinct and physiologically separable "components" which result from discrete mechanisms. An initial fast component is a sodium spike similar to that of squid nerve. The slow com- ponent, which accounts for both a slow depolarization during phase 0 and the plateau, probably is dependent on the properties of a slow inward current hav- ing a positive equilibrium potential, coupled to a decrease in the resting potas- sium conductance. -

V=Nx76xs 4FRE the Following Content Is Provided Under a Creative Commons License
MITOCW | watch?v=Nx76XS_4FRE The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To make a donation or view additional materials from hundreds of MIT courses, visit MIT OpenCourseWare at ocw.mit.edu. HAZEL SIVE: Some good questions. Let's zip through quickly. Why can't an initial depolarization travel along the length of the axon? Last time, we went through in detail how a signal is transmitted from the outside to the inside of the cell by ion flux across the membrane, and then how that ion flux is transmitted down the neuron. Remember that the ions entering the cell are just ions. They'll diffuse away. And so the depolarization kind of dissipates. And it then needs to be regenerated. The action potential, each small depolarization or the whole signal, each small depolarization with a specific magnitude, the reversal of membrane potential going from about minus 60 millivolts, negative inside, to plus 55 or 60 millivolts, positive inside. Each of those is an action potential. And here is an interesting one. Why doesn't the membrane potential stop at zero when sodium rushes into the cell during an action potential, which is what you'd expect if sodium was just moving along its concentration radian? Because the potential's not only governed by sodium influx. There are chloride ions outside the cell and potassium ions inside over the duration of the potential. And as we'll discuss briefly in a moment, it's during the rectification process that you go back to zero, and then you reverse membrane potential again. -

Electrophysiological Foundations of Cardiac Arrhythmias Clinical Electrophysiology Mark E
Second Edition Electrophysiological Foundations Andrew L. Wit of Cardiac Arrhythmias Penelope A. Boyden A Bridge Between Basic Mechanisms and Electrophysiological Foundations of Cardiac Arrhythmias Electrophysiological Foundations of Cardiac Clinical Electrophysiology Mark E. Josephson Second Edition Hein J. Wellens Now in an abridged second edition, Electrophysiological Foundations of Cardiac Arrhythmias focuses on teaching the fundamental concepts of cardiac cellular • Boyden Josephson Wellens Wit electrophysiology with an emphasis on the relationship of basic mechanisms to clinical cardiac arrhythmias. Understanding this relationship and the electrophysiological mechanisms underlying arrhythmogenesis will be invaluable Electrophysiological to physicians entering the fields of cardiology and clinical electrophysiology, as well as those scientists and clinicians already working in these areas. These essential concepts of electrophysiology include discussion on action potentials, ion channels and currents, and mechanisms of arrhythmias, and Foundations of provide the working knowledge that will enable the reader to approach a board exam confidently. Additionally, the authors build a base of understanding that will prepare the reader for more advanced texts, such as Josephson’s Clinical Cardiac Electrophysiology: Techniques and Interpretations. Cardiac Arrhythmias About the Authors The authors, Andrew L. Wit, PhD, Penelope A. Boyden, PhD, Mark E. Josephson, A Bridge Between MD, and Hein J. Wellens, MD, PhD, have spent four lifetimes investigating and defining electrophysiological mechanisms of cardiac arrhythmias. But equally important, they have devoted their careers to teaching this subject to students of all levels, from medical Basic Mechanisms and students to more advanced cardiology and clinical electrophysiology fellows. Clinical Electrophysiology ISBN 978-1-942909-42-2 Second Edition 90000> This book includes access to a free digital edition for use by the first buyer. -

1 the New Definition of Early Repolarisation Peter W. Macfarlane
1 The New Definition of Early Repolarisation Peter W. Macfarlane Institute of Cardiovascular and Medical Sciences University of Glasgow Address for Correspondence: Electrocardiology Group Level 1, New Lister Building Royal Infirmary, Glasgow G31 2ER Scotland, UK. e-mail: [email protected] 2 Introduction There has been an increasing interest in the electrocardiographic pattern of early repolarization in recent years following the publication of a paper by Haissaguerre and colleagues [1] in 2008, which linked their definition of the pattern to sudden cardiac arrest due to idiopathic ventricular fibrillation. However, earlier and later publications [2-17] by other groups on the topic of early repolarisation seemed to exhibit disagreement over what constituted the ECG pattern of early repolarisation suggesting a degree of confusion following the 2008 publication [1]. In 1947, Myers et al [2] reported that, among other features, an “elevation of the RS-T take off of 0.5 - 2.0mm may be considered as a normal variant” provided that the ST segment was concave upwards and followed by a tall T wave. In 1951, Goldman also reported on RS-T segment elevation in V3-V6 as a normal variant [3]. The original description of what for many years was regarded as the early repolarization pattern can probably be traced back to an early paper by Wasserburger and Alt [4] dealing with normal RS-T elevation in the relatively newly established full 12 lead ECG. The authors pointed out that the ST elevation mostly arose from a distinct notch on the downslope of the R wave but was occasionally associated with a “well-defined slur”. -

NEURON and the PHYSIOLOGY of NERVE IMPULSE the Nervous
NEURON AND THE PHYSIOLOGY OF NERVE IMPULSE The nervous system is made of millions of structural and functional units called nerve cell or neurons and a number of supporting cells called glial cells. The function of the nerve cell is to receive and transmit impulses or messages and integrate the entire body as a single unit. The neurons can be classified into three types – 1. Unipolar neuron- it has a cell body or cyton from which arises a single short branch called neurite that immediately divides into the dendritic or receiving branch and axonic or transmitting branch. 2. Bipolar neuron- The neuron has the cell body from which arises two neurites called the dendron and the axon respectively. 3. Multipolar neuron- From the cell body arise many dendrites and one long axon. Neurons can also be classified into 1) myelinated when myelin sheath surrounds the axon and 2) non-myelinated neurons when the axon is naked and not covered by the myelin sheath. 1 Structure of a myelinated neuron/nerve cell The neuron can be divided into three parts-cell body or cyton from which arises two types of branches, i.e., many branched processes called dendrons and a long unbranched process called axon. The cyton or cell body is filled with the cytoplasm also called neuroplasm that is limited by the plasma membrane. It contains the nucleus, mitochondria, endoplasmic reticulum, golgi bodies, neurofibrils (filaments that act as a cytoskeleton) and special dense RNA rich granules called Nissl’s granules (help in protein synthesis). The dendrons terminate in slender processes called dendrites.