Epithelial–Stromal Interactions in the Mouse and Human Mammary Gland in Vivo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Polythelia -Six Nipples in Amiddle Varun Arunagiri in the Accessory Nipples
Stanley Medical Journal CASE REPORT - GENERAL SURGERY Polythelia - Six Nipples in a middle aged woman Varun Arunagiri(1), Kothai Anbalagan(1) Abstract Vol 3 | Issue 2 | April - June | 2016 - June 2 | April 3 | Issue Vol Supernumerary nipples are more than two nipples which normally exist in humans. Polythelia or supernumerary nipple is a rare condition with higher prevalence in males than in females with the ratio of 1.7:1. The maximum reported number of nipples in a person with Polythelia is seven in a male. The usual presentation of Polythelia is with three nipples. Here is an image of Polythelia in a 40 year old female presenting with six nipples without lactation from the supernumer- ary nipples and any other anomalies. She has breast fed her two children. Key-words: Polythelia; Supernumerary Nipples; Mammary ridge; Kajava Classification; Clear cells of Toker. Key Messages: 1. Polythelia is a benign condition with chances of malignancy in the accessory nipples. 2. Constant follow-up is needed when the patient says lump in the region of accessory nipple. 3. Lactation during the pregnancy is common. INTRODUCTION: reported number of nipples in a person with Polythelia is seven in a male. The usual presentation of polythelia is with Polythelia is a congenital anomaly of the breast three nipples where in there are accessory nipples along the milk line Mammals have six to seven nipples, which are apart from the normal two nipples. Amazia, polymazia, common among canines and felines. It is rare to see humans Polythelia, athelia are few congenital anomalies of the nip- with more than three nipples. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Basic Histology (23 Questions): Oral Histology (16 Questions
Board Question Breakdown (Anatomic Sciences section) The Anatomic Sciences portion of part I of the Dental Board exams consists of 100 test items. They are broken up into the following distribution: Gross Anatomy (50 questions): Head - 28 questions broken down in this fashion: - Oral cavity - 6 questions - Extraoral structures - 12 questions - Osteology - 6 questions - TMJ and muscles of mastication - 4 questions Neck - 5 questions Upper Limb - 3 questions Thoracic cavity - 5 questions Abdominopelvic cavity - 2 questions Neuroanatomy (CNS, ANS +) - 7 questions Basic Histology (23 questions): Ultrastructure (cell organelles) - 4 questions Basic tissues - 4 questions Bone, cartilage & joints - 3 questions Lymphatic & circulatory systems - 3 questions Endocrine system - 2 questions Respiratory system - 1 question Gastrointestinal system - 3 questions Genitouirinary systems - (reproductive & urinary) 2 questions Integument - 1 question Oral Histology (16 questions): Tooth & supporting structures - 9 questions Soft oral tissues (including dentin) - 5 questions Temporomandibular joint - 2 questions Developmental Biology (11 questions): Osteogenesis (bone formation) - 2 questions Tooth development, eruption & movement - 4 questions General embryology - 2 questions 2 National Board Part 1: Review questions for histology/oral histology (Answers follow at the end) 1. Normally most of the circulating white blood cells are a. basophilic leukocytes b. monocytes c. lymphocytes d. eosinophilic leukocytes e. neutrophilic leukocytes 2. Blood platelets are products of a. osteoclasts b. basophils c. red blood cells d. plasma cells e. megakaryocytes 3. Bacteria are frequently ingested by a. neutrophilic leukocytes b. basophilic leukocytes c. mast cells d. small lymphocytes e. fibrocytes 4. It is believed that worn out red cells are normally destroyed in the spleen by a. neutrophils b. -
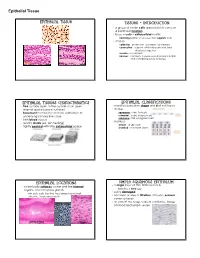
Epithelial Tissue
Epithelial Tissue Epithelial Tissue Tissues - Introduction · a group of similar cells specialized to carry on a particular function · tissue = cells + extracellular matrix nonliving portion of a tissue that supports cells · 4 types epithelial - protection, secretion, absorption connective - support soft body parts and bind structures together muscle - movement nervous - conducts impulses used to help control and coordinate body activities Epithelial Tissues Characteristics Epithelial Classifications · free surface open to the outside or an open · classified based on shape and # of cell layers internal space (apical surface) · shape · basement membrane anchors epithelium to squamous - thin, flat cells underlying connective tissue cuboidal - cube-shaped cells columnar - tall, elongated cells · lack blood vessels · number · readily divide (ex. skin healing) simple - single layer · tightly packed with little extracellular space stratified - 2 or more layers Epithelial Locations Simple Squamous Epithelium · a single layer of thin, flattened cells · cover body surfaces, cover and line internal organs, and compose glands looks like a fried egg · easily damaged skin cells, cells that line the stomach and small intestine, inside your mouth · common at sites of filtration, diffusion, osmosis; cover surfaces · air sacs of the lungs, walls of capillaries, linings cheek cells of blood and lymph vessels intestines skin Epithelial Tissue Simple Cuboidal Epithelium Simple Columnar Epithelium · single layer of cube-shaped cells · single layer of cells -

A Study of Evaluation and Management of Rare Congenital Breast Diseases Surgery Section
Original Article DOI: 10.7860/JCDR/2016/21077.8648 A Study of Evaluation and Management of Rare Congenital Breast Diseases Surgery Section RIKKI SINGAL1, SUDHIR KUMAR MEHTA2, JYOTI BALA3, MUZZAFAR ZAMAN4, AMIT MITTAL5, GUARAV GUPTA6, SAMER RUDRA7, SAMITA SINGAL8 ABSTRACT Results: Out of 32 cases: 1(3.125%) male patient had Introduction: Polymastia and polythelia may be asymptomatic unilateral and 1(3.125%) male had bilateral accessory nipple, or cause pain, restriction of arm movement, milk discharge, 7 (21.87%) females had unilateral and 1(3.125%) had bilateral cosmetic problems or anxiety. Cosmesis is the main indication accessory nipple, 1 (3.125%) diagnosed as accessory axillary for surgical excision of accessory breasts in axilla. In addition fibroadenoma in female, 16(50%) presented as unilateral and 5 it also confirms the diagnosis and allays the patient’s fear of (15.62%) had bilateral swelling in the axilla as accessory breast. harbouring a malignancy. Patients underwent surgical excision and in 8(25%) cases z- shaped incision was made in view of better cosmesis. Patients Aim: To evaluate the presentation of symptoms, investigations were followed up upto 6 months postoperatively. There were no required for diagnosis and the management to improve the residual swelling and movements of the arm over the shoulder treatment protocols in patients with breast diseases. joint were normal. In 3(9.37%) cases, wound dehiscence Materials and Methods: This retrospective study on breast occurred; in 2 (6.25%) cases lymphoedema formation was diseases presenting as supernumerary breasts and nipples seen. These were successfully managed conservatively. was conducted in the Department of Surgery between January Conclusion: As breast swellings either fibroadenoma or 2013 and January 2016 at MMIMS Research and hospital, carcinoma are common entities to come across everywhere Mullana, Ambala. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Diagnosis and Treatment of Accessory Breast Cancer in 11 Patients
ONCOLOGY LETTERS 10: 1783-1788, 2015 Diagnosis and treatment of accessory breast cancer in 11 patients SHUO ZHANG1,2*, YONG-HUA YU2, WEI QU2, YONG ZHANG2* and JIA LI2 1School of Medical and Life Sciences, Shandong Academy of Medical Sciences, Jinan University, Jinan, Shandong 250200; 2Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Jinan, Shandong 250117, P.R. China Received August 21, 2014; Accepted May 8, 2015 DOI: 10.3892/ol.2015.3388 Abstract. The present study aimed to investigate the clinical from the ectodermal ridges, also known as the milk lines, on characteristics, diagnosis and treatment of accessory breast the ventral surface of the body, which extend from the axillae cancer, and contribute valuable information regarding this to the inguinal regions and end on the medial aspect of the rare tumour to the current literature, ultimately facilitating thighs on each side of the body (4). Embryologically, ectopic the development of improved treatment strategies. The present breast tissue develops as a result of failed resolution of the study reported the cases of 11 patients with accessory breast mammary ridge, an ectodermal thickening that extends from cancer. The patients with accessory breast cancer were the axilla to the groin (5). Ectopic breast tissue may appear at admitted between January 2002 and June 2014, and the patient any site along the milk line, but it occurs most commonly in records were retrospectively analysed. All patients presented the axill; less commonly, it may appear in locations outside with a tumour that was localised in the axilla. Out of these of the mammary ridge, including the face, middle back, patients, there were 8 patients with invasive ductal carcinoma buttock, posterior neck, chest, vulva, hip, posterior, flank and 3 patients with invasive lobular carcinoma. -

Squamous Epithelium in the Human Thyroid Gland
J Clin Pathol: first published as 10.1136/jcp.19.4.384 on 1 July 1966. Downloaded from J. clin. Path. (1966), 19, 384. Squamous epithelium in the human thyroid gland J. N. HARCOURT-WEBSTER1 From the Department ofPathology, University ofEdinburgh SYNOPSIS Four cases are reported in each of which squamous epithelium was an incidental finding in surgically excised thyroid gland tissue. The occasional thyroid cyst lined throughout by squamous cells probably represents a persistent ultimo-branchial body, but the evidence indicates that the usual source of such cells in this gland is metaplasia of the follicular epithelium. An explanation is offered for the infrequency of this transformation in the thyroid, despite the frequent occurrence of the changes which predispose to epithelial metaplasia at other sites. There is no evidence to suggest that squamous cells arising in this gland by either of these means have any sinister significance. Squamous epithelium was first described in a human iodine uptake studies were normal but serological anti- thyroid gland by Nicholson (1922); he attributed body tests were positive (ADT: +ve on second day; this finding to metaplasia of the follicular epithelium TCH: 1/250,000; CFT: 1/250). The provisional diagnosis was Hashimoto's disease; a biopsy was taken from the induced by severe chronic inflammatory and fibrotic right lobe with a wedge resection of the isthmus. changes in the gland. Epithelial metaplasia occurs in Histology Sections of the rock-hard, pale fawn tissue response to altered function or at least as the result show abundant, interwoven, thick bands of hyaline col- of altered environment (Boyd, 1961). -
Anatomy of the Breast Doctors Notes Notes/Extra Explanation Please View Our Editing File Before Studying This Lecture to Check for Any Changes
Color Code Important Anatomy of the Breast Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives By the end of the lecture, the student should be able to: ✓ Describe the shape and position of the female breast. ✓ Describe the structure of the mammary gland. ✓ List the blood supply of the female breast. ✓ Describe the lymphatic drainage of the female breast. ✓ Describe the applied anatomy in the female breast. Highly recommended Introduction 06:26 Overview of the breast: • The breast (consists of mammary glands + associated skin & Extra connective tissue) is a gland made up of lobes arranged radially .around the nipple (شعاعيا) • Each lobe is further divided into lobules. Between the lobes and lobules we have fat & ligaments called ligaments of cooper • These ligaments attach the skin to the muscle (beneath the breast) to give support to the breast. in shape (مخروطي) *o Shape: it is conical o Position: It lies in superficial fascia of the front of chest. * o Parts: It has a: 1. Base lies on muscles, (حلمة الثدي) Apex nipple .2 3. Tail extend into axilla Extra Position of Female Breast (حلقة ملونة) Base Nipple Areola o Extends from 2nd to 6th ribs. o It extends from the lateral margin of sternum medially to the midaxillary line laterally. o It has no capsule. o It lies on 3 muscles: • 2/3 of its base on (1) pectoralis major* Extra muscle, • inferolateral 1/3 on (2) Serratus anterior & (3) External oblique muscles (muscle of anterior abdominal wall). o Its superolateral part sends a process into the axilla called the axillary tail or axillary process. -

Anatomy of the Human Mammary Gland: Current Status of Knowledge
Clinical Anatomy 00:000–000 (2012) REVIEW Anatomy of the Human Mammary Gland: Current Status of Knowledge 1,2 1 FOTEINI HASSIOTOU AND DONNA GEDDES * 1Hartmann Human Lactation Research Group, School of Chemistry and Biochemistry, Faculty of Science, The University of Western Australia, Crawley, Western Australia, Australia 2School of Anatomy, Physiology and Human Biology, Faculty of Science, The University of Western Australia, Crawley, Western Australia, Australia Mammary glands are unique to mammals, with the specific function of synthe- sizing, secreting, and delivering milk to the newborn. Given this function, it is only during a pregnancy/lactation cycle that the gland reaches a mature devel- opmental state via hormonal influences at the cellular level that effect drastic modifications in the micro- and macro-anatomy of the gland, resulting in remodeling of the gland into a milk-secretory organ. Pubertal and post-puber- tal development of the breast in females aids in preparing it to assume a func- tional state during pregnancy and lactation. Remarkably, this organ has the capacity to regress to a resting state upon cessation of lactation, and then undergo the same cycle of expansion and regression again in subsequent pregnancies during reproductive life. This plasticity suggests tight hormonal regulation, which is paramount for the normal function of the gland. This review presents the current status of knowledge of the normal macro- and micro-anatomy of the human mammary gland and the distinct changes it undergoes during the key developmental stages that characterize it, from em- bryonic life through to post-menopausal age. In addition, it discusses recent advances in our understanding of the normal function of the breast during lac- tation, with special reference to breastmilk, its composition, and how it can be utilized as a tool to advance knowledge on normal and aberrant breast devel- opment and function. -

Mixed Type Adrenal Cyst: a Case Report
Eastern Journal of Medicine 20 (2015) 163-166 Case Report Mixed type adrenal cyst: A case report Gulay Buluta,*, Mehmet Deniz Bulutb, Deniz Yilmaza, Sebahattin Celikc, Nursen Toprakb aDepartment of Pathology, Yuzuncu Yil University Faculty of Medicine, Van, Turkey bDepartment of Radiology, Van Research and Training Hospital, Van, Turkey cDepartment of General Surgery, Van Research and Training Hospital, Van, Turkey Abstract. Adrenal cysts are rare lesions that usually progress asymptomatically. They are often determined post- mortem. Our case was a 50-year old-male who had presented to our clinic with upper right quadrant pain. The computed tomography demonstrated a cystic mass in the adrenal gland. No abnormalities were determined in the laboratory tests. According to the histopathological and immunohistochemical examination, the cyst was evaluated as a mixed-type adrenal cyst, the wall of which was covered with endothelium, mesothelium and cubic epithelium. We believed that the case was worth presenting, since it was the first diagnosed mixed-type adrenal cyst in the literature. Key words: Mixed-type adrenal cyst, endothelial cyst, epithelial cyst, mesothelial cyst pain. There was no history of hypertension or 1. Introduction trauma in the past. Per abdominal examination Adrenal cysts are rarely encountered clinical showed a soft abdomen; no mass was felt. entities. Their incidence has been reported from Haemogram was normal. A complete endocrine 0.06%-0.18% in autopsy series (1). In 1966, workup including the estimation of urinary Foster put forth a classification based on the metanephrines, vanillylmandelic acid and free histopathology of 220 adrenal cysts into parasitic cortisol did not show any hormonal cysts (7%), epithelial cysts (9%), pseudocysts hypersecretion. -

12. Development of Axial Skeleton and Extremities. Muscles and Skin
Z. Tonar, M. Králíčková: Outlines of lectures on embryology for 2 nd year students of General medicine and Dentistry License Creative Commons - http://creativecommons.org/licenses/by-nc-nd/3.0/ 12. Development of axial skeleton and extremities. Muscles and skin. Timeline − 19 days: somites emerge in the gastrula − 4 weeks: sclerotome cells migrate along the neural tube − 5 weeks: mesenchymal blastema of the axial skeleton − 6 weeks: mesenchymal blastema of limbs − 8 weeks: rotation of the limbs − 8 weeks: individual muscles differentiate − 10 weeks: primary ossification centres in diaphyses − 3 months: bones of the skull develop − 9 months: diaphyses ossified; secondary ossification centres emerge In general, bone tissue originates from: − the of the somitic paraxial mesoderm, namely from the ventromedial part, the sclerotome − the head non-segmented mesoderm − the somatopleuric lateral plate mesoderm skeleton of limbs − the neural crest, which differentiates into the head ectomesenchyme − mesenchyme o its cells migrate and differentiate into fibroblasts, the source of the desmogenous (intramembranous) ossification o its cells imgrate and differentiate into chondroblasts, the source of the chondrogenous ossification of the hyaline cartilage models Limbs − week 4: limb buds o somatopleuric mesenchyme differentiates into bones and connective tissues o myogenic cells, angioblasts and nerves grow in o surface ectoderm thickens into the apical ectodermal ridge − week 6: each limb is divided proximodistally into three components: o autopod