Coulomb's Law - Wikipedia, the Free Encyclopedia Page 1 of 7
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Measurement of the Speed of Gravity
Measurement of the Speed of Gravity Yin Zhu Agriculture Department of Hubei Province, Wuhan, China Abstract From the Liénard-Wiechert potential in both the gravitational field and the electromagnetic field, it is shown that the speed of propagation of the gravitational field (waves) can be tested by comparing the measured speed of gravitational force with the measured speed of Coulomb force. PACS: 04.20.Cv; 04.30.Nk; 04.80.Cc Fomalont and Kopeikin [1] in 2002 claimed that to 20% accuracy they confirmed that the speed of gravity is equal to the speed of light in vacuum. Their work was immediately contradicted by Will [2] and other several physicists. [3-7] Fomalont and Kopeikin [1] accepted that their measurement is not sufficiently accurate to detect terms of order , which can experimentally distinguish Kopeikin interpretation from Will interpretation. Fomalont et al [8] reported their measurements in 2009 and claimed that these measurements are more accurate than the 2002 VLBA experiment [1], but did not point out whether the terms of order have been detected. Within the post-Newtonian framework, several metric theories have studied the radiation and propagation of gravitational waves. [9] For example, in the Rosen bi-metric theory, [10] the difference between the speed of gravity and the speed of light could be tested by comparing the arrival times of a gravitational wave and an electromagnetic wave from the same event: a supernova. Hulse and Taylor [11] showed the indirect evidence for gravitational radiation. However, the gravitational waves themselves have not yet been detected directly. [12] In electrodynamics the speed of electromagnetic waves appears in Maxwell equations as c = √휇0휀0, no such constant appears in any theory of gravity. -
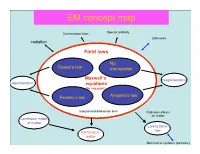
EM Concept Map
EM concept map Conservation laws Special relativity EM waves radiation Field laws No Gauss’s law monopoles ConservationMaxwell’s laws magnetostatics electrostatics equations (In vacuum) Faraday’s law Ampere’s law Integral and differential form Radiation effects on matter Continuum model of matter Lorenz force law EM fields in matter Mechanical systems (particles) Electricity and Magnetism PHYS-350: (updated Sept. 9th, (1605-1725 Monday, Wednesday, Leacock 109) 2010) Instructor: Shaun Lovejoy, Rutherford Physics, rm. 213, local Outline: 6537, email: [email protected]. 1. Vector Analysis: Tutorials: Tuesdays 4:15pm-5:15pm, location: the “Boardroom” Algebra, differential and integral calculus, curvilinear coordinates, (the southwest corner, ground floor of Rutherford physics). Office Hours: Thursday 4-5pm (either Lhermitte or Gervais, see Dirac function, potentials. the schedule on the course site). 2. Electrostatics: Teaching assistant: Julien Lhermitte, rm. 422, local 7033, email: Definitions, basic notions, laws, divergence and curl of the electric [email protected] potential, work and energy. Gervais, Hua Long, ERP-230, [email protected] 3. Special techniques: Math background: Prerequisites: Math 222A,B (Calculus III= Laplace's equation, images, seperation of variables, multipole multivariate calculus), 223A,B (Linear algebra), expansion. Corequisites: 314A (Advanced Calculus = vector 4. Electrostatic fields in matter: calculus), 315A (Ordinary differential equations) Polarization, electric displacement, dielectrics. Primary Course Book: "Introduction to Electrodynamics" by D. 5. Magnetostatics: J. Griffiths, Prentice-Hall, (1999, third edition). Lorenz force law, Biot-Savart law, divergence and curl of B, vector Similar books: potentials. -“Electromagnetism”, G. L. Pollack, D. R. Stump, Addison and 6. Magnetostatic fields in matter: Wesley, 2002. -

Analysis on Electric Field Based on Three Dimensional Atmospheric Electric Field Apparatus
J Electr Eng Technol.2018; 13(4): 1697-1704 ISSN(Print) 1975-0102 http://doi.org/10.5370/JEET.2018.13.4.1697 ISSN(Online) 2093-7423 Analysis on Electric Field Based on Three Dimensional Atmospheric Electric Field Apparatus Hong-yan Xing†, Gui-xian He* and Xin-yuan Ji** Abstract – As a key component of lighting location system (LLS) for lightning warning, the atmospheric electric field measuring is required to have high accuracy. The Conventional methods of the existent electric field measurement meter can only detect the vertical component of the atmospheric electric field, which cannot acquire the realistic electric field in the thunderstorm. This paper proposed a three dimensional (3D) electric field system for atmospheric electric field measurement, which is capable of three orthogonal directions in X, Y, Z, measuring. By analyzing the relationship between the electric field and the relative permittivity of ground surface, the permittivity is calculated, and an efficiency 3D measurement model is derived. On this basis, a three-dimensional electric field sensor and a permittivity sensor are adopted to detect the spatial electric field. Moreover, the elevation and azimuth of the detected target are calculated, which reveal the location information of the target. Experimental results show that the proposed 3D electric field meter has satisfactory sensitivity to the three components of electric field. Additionally, several observation results in the fair and thunderstorm weather have been presented. Keywords: Three dimension electric field, Permittivity sensor, Lightning, Electric field analysis. 1. Introduction cloud, which changes with the clouds changes [8]. So, there will be an existence of horizontal components of Monitoring of the atmosphere electric field is an the electric field other than the vertical component. -

Classical Mechanics
Classical Mechanics Hyoungsoon Choi Spring, 2014 Contents 1 Introduction4 1.1 Kinematics and Kinetics . .5 1.2 Kinematics: Watching Wallace and Gromit ............6 1.3 Inertia and Inertial Frame . .8 2 Newton's Laws of Motion 10 2.1 The First Law: The Law of Inertia . 10 2.2 The Second Law: The Equation of Motion . 11 2.3 The Third Law: The Law of Action and Reaction . 12 3 Laws of Conservation 14 3.1 Conservation of Momentum . 14 3.2 Conservation of Angular Momentum . 15 3.3 Conservation of Energy . 17 3.3.1 Kinetic energy . 17 3.3.2 Potential energy . 18 3.3.3 Mechanical energy conservation . 19 4 Solving Equation of Motions 20 4.1 Force-Free Motion . 21 4.2 Constant Force Motion . 22 4.2.1 Constant force motion in one dimension . 22 4.2.2 Constant force motion in two dimensions . 23 4.3 Varying Force Motion . 25 4.3.1 Drag force . 25 4.3.2 Harmonic oscillator . 29 5 Lagrangian Mechanics 30 5.1 Configuration Space . 30 5.2 Lagrangian Equations of Motion . 32 5.3 Generalized Coordinates . 34 5.4 Lagrangian Mechanics . 36 5.5 D'Alembert's Principle . 37 5.6 Conjugate Variables . 39 1 CONTENTS 2 6 Hamiltonian Mechanics 40 6.1 Legendre Transformation: From Lagrangian to Hamiltonian . 40 6.2 Hamilton's Equations . 41 6.3 Configuration Space and Phase Space . 43 6.4 Hamiltonian and Energy . 45 7 Central Force Motion 47 7.1 Conservation Laws in Central Force Field . 47 7.2 The Path Equation . -

High Performance Triboelectric Nanogenerator and Its Applications
HIGH PERFORMANCE TRIBOELECTRIC NANOGENERATOR AND ITS APPLICATIONS A Dissertation Presented to The Academic Faculty by Changsheng Wu In Partial Fulfillment of the Requirements for the Degree DOCTOR OF PHILOSOPHY in the SCHOOL OF MATERIALS SCIENCE AND ENGINEERING Georgia Institute of Technology AUGUST 2019 COPYRIGHT © 2019 BY CHANGSHENG WU HIGH PERFORMANCE TRIBOELECTRIC NANOGENERATOR AND ITS APPLICATIONS Approved by: Dr. Zhong Lin Wang, Advisor Dr. C. P. Wong School of Materials Science and School of Materials Science and Engineering Engineering Georgia Institute of Technology Georgia Institute of Technology Dr. Meilin Liu Dr. Younan Xia School of Materials Science and Department of Biomedical Engineering Engineering Georgia Institute of Technology Georgia Institute of Technology Dr. David L. McDowell School of Materials Science and Engineering Georgia Institute of Technology Date Approved: [April 25, 2019] To my family and friends ACKNOWLEDGEMENTS Firstly, I would like to express my sincere gratidue to my advisor Prof. Zhong Lin Wang for his continuous support and invaluable guidance in my research. As an exceptional researcher, he is my role model for his thorough knowledge in physics and nanotechnology, indefatigable diligence, and overwhelming passion for scientific innovation. It is my great fortune and honor in having him as my advisor and learning from him in the past four years. I would also like to thank the rest of my committee members, Prof. Liu, Prof. McDowell, Prof. Wong, and Prof. Xia for their insightful advice on my doctoral research and dissertation. My sincere thanks also go to my fellow lab mates for their strong support and help. In particular, I would not be able to start my research so smoothly without the mentorship of Dr. -

The Lorentz Force
CLASSICAL CONCEPT REVIEW 14 The Lorentz Force We can find empirically that a particle with mass m and electric charge q in an elec- tric field E experiences a force FE given by FE = q E LF-1 It is apparent from Equation LF-1 that, if q is a positive charge (e.g., a proton), FE is parallel to, that is, in the direction of E and if q is a negative charge (e.g., an electron), FE is antiparallel to, that is, opposite to the direction of E (see Figure LF-1). A posi- tive charge moving parallel to E or a negative charge moving antiparallel to E is, in the absence of other forces of significance, accelerated according to Newton’s second law: q F q E m a a E LF-2 E = = 1 = m Equation LF-2 is, of course, not relativistically correct. The relativistically correct force is given by d g mu u2 -3 2 du u2 -3 2 FE = q E = = m 1 - = m 1 - a LF-3 dt c2 > dt c2 > 1 2 a b a b 3 Classically, for example, suppose a proton initially moving at v0 = 10 m s enters a region of uniform electric field of magnitude E = 500 V m antiparallel to the direction of E (see Figure LF-2a). How far does it travel before coming (instanta> - neously) to rest? From Equation LF-2 the acceleration slowing the proton> is q 1.60 * 10-19 C 500 V m a = - E = - = -4.79 * 1010 m s2 m 1.67 * 10-27 kg 1 2 1 > 2 E > The distance Dx traveled by the proton until it comes to rest with vf 0 is given by FE • –q +q • FE 2 2 3 2 vf - v0 0 - 10 m s Dx = = 2a 2 4.79 1010 m s2 - 1* > 2 1 > 2 Dx 1.04 10-5 m 1.04 10-3 cm Ϸ 0.01 mm = * = * LF-1 A positively charged particle in an electric field experiences a If the same proton is injected into the field perpendicular to E (or at some angle force in the direction of the field. -

Gauss' Linking Number Revisited
October 18, 2011 9:17 WSPC/S0218-2165 134-JKTR S0218216511009261 Journal of Knot Theory and Its Ramifications Vol. 20, No. 10 (2011) 1325–1343 c World Scientific Publishing Company DOI: 10.1142/S0218216511009261 GAUSS’ LINKING NUMBER REVISITED RENZO L. RICCA∗ Department of Mathematics and Applications, University of Milano-Bicocca, Via Cozzi 53, 20125 Milano, Italy [email protected] BERNARDO NIPOTI Department of Mathematics “F. Casorati”, University of Pavia, Via Ferrata 1, 27100 Pavia, Italy Accepted 6 August 2010 ABSTRACT In this paper we provide a mathematical reconstruction of what might have been Gauss’ own derivation of the linking number of 1833, providing also an alternative, explicit proof of its modern interpretation in terms of degree, signed crossings and inter- section number. The reconstruction presented here is entirely based on an accurate study of Gauss’ own work on terrestrial magnetism. A brief discussion of a possibly indepen- dent derivation made by Maxwell in 1867 completes this reconstruction. Since the linking number interpretations in terms of degree, signed crossings and intersection index play such an important role in modern mathematical physics, we offer a direct proof of their equivalence. Explicit examples of its interpretation in terms of oriented area are also provided. Keywords: Linking number; potential; degree; signed crossings; intersection number; oriented area. Mathematics Subject Classification 2010: 57M25, 57M27, 78A25 1. Introduction The concept of linking number was introduced by Gauss in a brief note on his diary in 1833 (see Sec. 2 below), but no proof was given, neither of its derivation, nor of its topological meaning. Its derivation remained indeed a mystery. -

Optical Properties of Bulk and Nano
Lecture 3: Optical Properties of Bulk and Nano 5 nm Course Info First H/W#1 is due Sept. 10 The Previous Lecture Origin frequency dependence of χ in real materials • Lorentz model (harmonic oscillator model) e- n(ω) n' n'' n'1= ω0 + Nucleus ω0 ω Today optical properties of materials • Insulators (Lattice absorption, color centers…) • Semiconductors (Energy bands, Urbach tail, excitons …) • Metals (Response due to bound and free electrons, plasma oscillations.. ) Optical properties of molecules, nanoparticles, and microparticles Classification Matter: Insulators, Semiconductors, Metals Bonds and bands • One atom, e.g. H. Schrödinger equation: E H+ • Two atoms: bond formation ? H+ +H Every electron contributes one state • Equilibrium distance d (after reaction) Classification Matter ~ 1 eV • Pauli principle: Only 2 electrons in the same electronic state (one spin & one spin ) Classification Matter Atoms with many electrons Empty outer orbitals Outermost electrons interact Form bands Partly filled valence orbitals Filled Energy Inner Electrons in inner shells do not interact shells Do not form bands Distance between atoms Classification Matter Insulators, semiconductors, and metals • Classification based on bandstructure Dispersion and Absorption in Insulators Electronic transitions No transitions Atomic vibrations Refractive Index Various Materials 3.4 3.0 2.0 Refractive index: n’ index: Refractive 1.0 0.1 1.0 10 λ (µm) Color Centers • Insulators with a large EGAP should not show absorption…..or ? • Ion beam irradiation or x-ray exposure result in beautiful colors! • Due to formation of color (absorption) centers….(Homework assignment) Absorption Processes in Semiconductors Absorption spectrum of a typical semiconductor E EC Phonon Photon EV ωPhonon Excitons: Electron and Hole Bound by Coulomb Analogy with H-atom • Electron orbit around a hole is similar to the electron orbit around a H-core • 1913 Niels Bohr: Electron restricted to well-defined orbits n = 1 + -13.6 eV n = 2 n = 3 -3.4 eV -1.51 eV me4 13.6 • Binding energy electron: =−=−=e EB 2 2 eV, n 1,2,3,.. -

On the Covariant Representation of Integral Equations of the Electromagnetic Field
On the covariant representation of integral equations of the electromagnetic field Sergey G. Fedosin PO box 614088, Sviazeva str. 22-79, Perm, Perm Krai, Russia E-mail: [email protected] Gauss integral theorems for electric and magnetic fields, Faraday’s law of electromagnetic induction, magnetic field circulation theorem, theorems on the flux and circulation of vector potential, which are valid in curved spacetime, are presented in a covariant form. Covariant formulas for magnetic and electric fluxes, for electromotive force and circulation of the vector potential are provided. In particular, the electromotive force is expressed by a line integral over a closed curve, while in the integral, in addition to the vortex electric field strength, a determinant of the metric tensor also appears. Similarly, the magnetic flux is expressed by a surface integral from the product of magnetic field induction by the determinant of the metric tensor. A new physical quantity is introduced – the integral scalar potential, the rate of change of which over time determines the flux of vector potential through a closed surface. It is shown that the commonly used four-dimensional Kelvin-Stokes theorem does not allow one to deduce fully the integral laws of the electromagnetic field and in the covariant notation requires the addition of determinant of the metric tensor, besides the validity of the Kelvin-Stokes theorem is limited to the cases when determinant of metric tensor and the contour area are independent from time. This disadvantage is not present in the approach that uses the divergence theorem and equation for the dual electromagnetic field tensor. -

Capacitance and Dielectrics Capacitance
Capacitance and Dielectrics Capacitance General Definition: C === q /V Special case for parallel plates: εεε A C === 0 d Potential Energy • I must do work to charge up a capacitor. • This energy is stored in the form of electric potential energy. Q2 • We showed that this is U === 2C • Then we saw that this energy is stored in the electric field, with a volume energy density 1 2 u === 2 εεε0 E Potential difference and Electric field Since potential difference is work per unit charge, b ∆∆∆V === Edx ∫∫∫a For the parallel-plate capacitor E is uniform, so V === Ed Also for parallel-plate case Gauss’s Law gives Q Q εεε0 A E === σσσ /εεε0 === === Vd so C === === εεε0 A V d Spherical example A spherical capacitor has inner radius a = 3mm, outer radius b = 6mm. The charge on the inner sphere is q = 2 C. What is the potential difference? kq From Gauss’s Law or the Shell E === Theorem, the field inside is r 2 From definition of b kq 1 1 V === dr === kq −−− potential difference 2 ∫∫∫a r a b 1 1 1 1 === 9 ×××109 ××× 2 ×××10−−−9 −−− === 18 ×××103 −−− === 3 ×××103 V −−−3 −−−3 3 ×××10 6 ×××10 3 6 What is the capacitance? C === Q /V === 2( C) /(3000V ) === 7.6 ×××10−−−4 F A capacitor has capacitance C = 6 µF and charge Q = 2 nC. If the charge is Q.25-1 increased to 4 nC what will be the new capacitance? (1) 3 µF (2) 6 µF (3) 12 µF (4) 24 µF Q. -

Units and Magnitudes (Lecture Notes)
physics 8.701 topic 2 Frank Wilczek Units and Magnitudes (lecture notes) This lecture has two parts. The first part is mainly a practical guide to the measurement units that dominate the particle physics literature, and culture. The second part is a quasi-philosophical discussion of deep issues around unit systems, including a comparison of atomic, particle ("strong") and Planck units. For a more extended, profound treatment of the second part issues, see arxiv.org/pdf/0708.4361v1.pdf . Because special relativity and quantum mechanics permeate modern particle physics, it is useful to employ units so that c = ħ = 1. In other words, we report velocities as multiples the speed of light c, and actions (or equivalently angular momenta) as multiples of the rationalized Planck's constant ħ, which is the original Planck constant h divided by 2π. 27 August 2013 physics 8.701 topic 2 Frank Wilczek In classical physics one usually keeps separate units for mass, length and time. I invite you to think about why! (I'll give you my take on it later.) To bring out the "dimensional" features of particle physics units without excess baggage, it is helpful to keep track of powers of mass M, length L, and time T without regard to magnitudes, in the form When these are both set equal to 1, the M, L, T system collapses to just one independent dimension. So we can - and usually do - consider everything as having the units of some power of mass. Thus for energy we have while for momentum 27 August 2013 physics 8.701 topic 2 Frank Wilczek and for length so that energy and momentum have the units of mass, while length has the units of inverse mass. -

Coulomb's Force
COULOMB’S FORCE LAW Two point charges Multiple point charges Attractive Repulsive - - + + + The force exerted by one point charge on another acts along the line joining the charges. It varies inversely as the square of the distance separating the charges and is proportional to the product of the charges. The force is repulsive if the charges have the same sign and attractive if the charges have opposite signs. q1 Two point charges q1 and q2 q2 [F]-force; Newtons {N} [q]-charge; Coulomb {C} origin [r]-distance; meters {m} [ε]-permittivity; Farad/meter {F/m} Property of the medium COULOMB FORCE UNIT VECTOR Permittivity is a property of the medium. Also known as the dielectric constant. Permittivity of free space Coulomb’s constant Permittivity of a medium Relative permittivity For air FORCE IN MEDIUM SMALLER THAN FORCE IN VACUUM Insert oil drop Viewing microscope Eye Metal plates Millikan oil drop experiment Charging by contact Example (Question): A negative point charge of 1µC is situated in air at the origin of a rectangular coordinate system. A second negative point charge of 100µC is situated on the positive x axis at the distance of 500 mm from the origin. What is the force on the second charge? Example (Solution): A negative point charge of 1µC is situated in air at the origin of a rectangular coordinate system. A second negative point charge of 100µC is situated on the positive x axis at the distance of 500 mm from the origin. What is the force on the second charge? Y q1 = -1 µC origin X q2= -100 µC Example (Solution): Y q1 = -1 µC origin X q2= -100 µC END Multiple point charges It has been confirmed experimentally that when several charges are present, each exerts a force given by on every other charge.