Gestation-Specific Reference Intervals for Comprehensive Spot Urinary Steroid Hormone Metabolite Analysis in Normal Singleton Pregnancy and 6 Weeks Postpartum Hiten D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States July 2016 2 Table of Contents
Deuterium Labelled Compounds United States July 2016 2 Table of Contents International Distributors 3 Corporate Overview 4 General Information 5 Pricing and Payment 5 Quotations 5 Custom Synthesis 5 Shipping 5 Quality Control 6 Quotations 6 Custom Synthesis 6 Shipping 6 Quality Control 6 Chemical Abstract Service Numbers 6 Handling Hazardous Compounds 6 Our Products are Not Intended for Use in Humans 7 Limited Warranty 7 Packaging Information 7 Alphabetical Listings 8 Stock Clearance 236 Products by Category 242 n-Alkanes 243 α-Amino Acids, N-Acyl α-Amino Acids, N-t-BOC Protected α-Amino Acid 243 and N-FMOC Protected α-Amino Acids Buffers and Reagents for NMR Studies 245 Detergents 245 Environmental Standards 246 Fatty Acids and Fatty Acid Esters 249 Flavours and Fragrances 250 Gases 253 Medical Research Products 254 Nucleic Acid Bases and Nucleosides 255 Pesticides and Pesticide Metabolites 256 Pharmaceutical Standards 257 Polyaromatic Hydrocarbons (PAHs), Alkyl-PAHs, Amino-PAHs, 260 Hydroxy-PAHs and Nitro-PAHs Polychlorinated Biphenyls (PCBs) 260 Spin Labels 261 Steroids 261 3 International Distributors C Beijng Zhenxiang H EQ Laboratories GmbH Australia K Technology Company Graf-von-Seyssel-Str. 10 Rm. 15A01, Changyin Bld. 86199 Augsburg Austria H No. 88, YongDingLu Rd. Germany Beijing 100039 Tel.: (49) 821 71058246 Belgium J China Fax: (49) 821 71058247 Tel.: (86) 10-58896805 [email protected] China C Fax: (86) 10-58896158 www.eqlabs.de Czech Republic H [email protected] Germany, Austria, China Czech Republic, Greece, Denmark I Hungary, -
Hormone Testing Summary
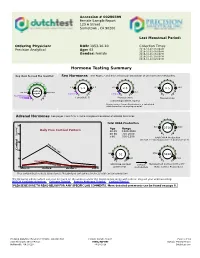
Accession # 00280399 Female Sample Report 123 A Street Sometown , CA 90266 Last Menstrual Period: Ordering Physician: DOB: 1953-10-10 Collection Times: Precision Analytical Age: 63 2016-10-02 06:00AM 2016-10-02 08:00AM Gender: Female 2016-10-01 06:00PM 2016-10-01 10:00PM 2016-10-02 02:00AM Hormone Testing Summary Key (how to read the results): Sex Hormones See Pages 2 and 3 for a thorough breakdown of sex hormone metabolites opausa n l R e a m n e g 1.8 4.5 6.0 2.3 14.0 r 5.1 2.8 1.5 P e patient low limit high limit 20.0 result 0.2-0.7 0.3-2.0 Postmenopausal range Estradiol(E2) Progesterone Testosterone (Serum Equivalent, ng/mL) Progesterone Serum Equivalent is a calculated value based on urine pregnanediol. Adrenal Hormones See pages 4 and 5 for a more complete breakdown of adrenal hormones Total DHEA Production 300 500 3000 Age Range 2516 Daily Free Cortisol Pattern 20-39 1300-3000 240 40-60 750-2000 (ng/mg) >60 500-1200 Total DHEA Production (DHEAS + Etiocholanolone + Androsterone) 180 High Range Limit 120Cortisol 80 52 2750 5930 60 230 6500 Patient Values Low Range Limit 24hr Free Cortisol cortisol Metabolized Cortisol (THF+THE) 0 (A+B+C+D) metabolism (Total Cortisol Production) Waking (A) Morning (B) Afternoon (C) Night (D) Free cortisol best reflects tissue levels. Metabolized cortisol best reflects total cortisol production. The following videos (which can also be found on the website under the listed names along with others) may aid your understanding: DUTCH Complete Overview Estrogen Tutorial Female Androgen Tutorial Cortisol Tutorial PLEASE BE SURE TO READ BELOW FOR A NY SPECIFIC LA B COMMENTS. -

The Steroid Metabolome in Men with Mood and Anxiety Disorders
Physiol. Res. 64 (Suppl. 2): S275-S282, 2015 https://doi.org/10.33549/physiolres.933067 The Steroid Metabolome in Men With Mood and Anxiety Disorders M. DUŠKOVÁ1, M. HILL1, M. BIČÍKOVÁ1, M. ŠRÁMKOVÁ1, D. ŘÍPOVÁ2, P. MOHR2, L. STÁRKA1 1Institute of Endocrinology, Prague, Czech Republic, 2National Institute of Mental Health, Klecany, Czech Republic Received May 5, 2015 Accepted May 20, 2015 Summary many other hormones and various factors have been The mood and behavior of individuals result from an orchestra of identified as modulators of mood and behavior in such many factors. Among them steroids play an important role; disorders. Recent studies have described the role of brain- however, only several common hormones have been investigated derived neurotrophic factor (BDNF) (Pluchino et al. in this respect. It has been demonstrated that some steroid 2013, Numakawa et al. 2014), thyroid hormones (Duntas metabolites long considered merely the products of steroid and Mails 2013), inflammation (Halaris 2013), immunity hormone metabolism in fact possess considerable activity in the (Pitychoutis and Papadopoulou-Daifoti 2010), melatonin CNS. For this reason we studied the steroid metabolome (Boyce and Hopwood 2013), oxytocin and vasopressin including 50 analytes in 20 men with depression, 20 men with (Scantamburlo et al. 2009, Matsuzaki et al. 2012), the anxiety and 30 healthy controls. Significant differences were renin-angiotensin-aldosterone system (Franklin et al. found not only between controls and men with either depression 2012, Murck et al. 2012), the cannabinoid system or anxiety, but also between men with depression and anxiety. (Martykánová 2010, Gorzalka and Hill 2011, Smaga et Particularly striking were those steroids until now not generally al. -

Increased and Mistimed Sex Hormone Production in Night Shift Workers
Published OnlineFirst March 3, 2015; DOI: 10.1158/1055-9965.EPI-14-1271 Research Article Cancer Epidemiology, Biomarkers Increased and Mistimed Sex Hormone Production & Prevention in Night Shift Workers Kyriaki Papantoniou1,2,3,4, Oscar J. Pozo2, Ana Espinosa1,2,3,4, Josep Marcos2,3, Gemma Castano-Vinyals~ 1,2,3,4, Xavier Basagana~ 1,2,3,4, Elena Juanola Pages 5, Joan Mirabent6,7, Jordi Martín8, Patricia Such Faro9, Amparo Gasco Aparici10, Benita Middleton11, Debra J. Skene11, and Manolis Kogevinas1,2,3,4,12 Abstract Background: Night shift work has been associated with Results: Night workers had higher levels of total progestagens an increased risk for breast and prostate cancer. The effect [geometric mean ratio (GMR) 1.65; 95% confidence intervals of circadian disruption on sex steroid production is a pos- (CI), 1.17–2.32] and androgens (GMR: 1.44; 95% CI, 1.03–2.00), sible underlying mechanism, underinvestigated in hum- compared with day workers, after adjusting for potential con- ans. We have assessed daily rhythms of sex hormones founders. The increased sex hormone levels among night and melatonin in night and day shift workers of both shift workers were not related to the observed suppression of sexes. 6-sulfatoxymelatonin. Peak time of androgens was significantly Methods: We recruited 75 night and 42 day workers, ages later among night workers, compared with day workers (testos- 22 to 64 years, in different working settings. Participants terone: 12:14 hours; 10:06-14:48 vs. 08:35 hours; 06:52-10:46). collected urine samples from all voids over 24 hours on a Conclusions: We found increased levels of progestagens and working day. -

A New Robust Technique for Testing of Glucocorticosteroids in Dogs and Horses Terry E
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2007 A new robust technique for testing of glucocorticosteroids in dogs and horses Terry E. Webster Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Veterinary Toxicology and Pharmacology Commons Recommended Citation Webster, Terry E., "A new robust technique for testing of glucocorticosteroids in dogs and horses" (2007). Retrospective Theses and Dissertations. 15029. https://lib.dr.iastate.edu/rtd/15029 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. A new robust technique for testing of glucocorticosteroids in dogs and horses by Terry E. Webster A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Toxicology Program o f Study Committee: Walter G. Hyde, Major Professor Steve Ensley Thomas Isenhart Iowa State University Ames, Iowa 2007 Copyright © Terry Edward Webster, 2007. All rights reserved UMI Number: 1446027 Copyright 2007 by Webster, Terry E. All rights reserved. UMI Microform 1446027 Copyright 2007 by ProQuest Information and Learning Company. All rights reserved. This microform edition is protected against unauthorized copying under Title 17, United States Code. ProQuest Information and Learning Company 300 North Zeeb Road P.O. Box 1346 Ann Arbor, MI 48106-1346 ii DEDICATION I want to dedicate this project to my wife, Jackie, and my children, Shauna, Luke and Jake for their patience and understanding without which this project would not have been possible. -

Studies on Steroid Fever II. Pyrogenic and Anti-Pyrogenic Activity in Vitro of Some Endogenous Steroids of Man
Studies on steroid fever II. Pyrogenic and anti-pyrogenic activity in vitro of some endogenous steroids of man G. M. Dillard, Phyllis Bodel J Clin Invest. 1970;49(12):2418-2426. https://doi.org/10.1172/JCI106461. Research Article The pyrogenic properties of some C-19 and C-21 steroids were examined by in vitro incubation of human blood leukocytes with serum-buffer solutions of the steroids and injection of the 18-hr supernatants into rabbits. In previous studies this method demonstrated release of leukocyte endogenous pyrogen by etiocholanolone. With two exceptions, steroids known to cause fever in man, such as 11β-OH etiocholanolone and 3α-hydroxy-5β-pregnane-20-one were also pyrogenic in vitro. All steroids tested which are nonpyrogenic in man, such as androsterone, 3β-OH etiocholanolone, and 3α, 17α-dihydroxy-5β-pregnan-20-one were also nonpyrogenic in vitro. Solubility in aqueous solution did not correlate with pyrogenic capacity. Inhibition of pyrogen release from human leukocytes in vitro by hydrocortisone and estradiol was demonstrated. Hydrocortisone-treated leukocytes released less pyrogen than did normal leukocytes when stimulated either by etiocholanolone or by phagocytosis of heat-killed staphylococci. On the other hand, estradiol-treated blood leukocytes and mononuclear cells showed significant suppression of pyrogen release when phagocytosis, but not etiocholanolone, was used as the stimulus. When blood cells were incubated with progesterone, greater than normal amounts of pyrogen were released following phagocytosis, and the inhibiting effect of estradiol could be partially reversed. Neither estradiol nor hydrocortisone appeared to act on rabbit leukocytes. These studies indicate that a variety of naturally-occurring steroids may alter pyrogen release from leukocytes. -

A Thesis Entitled "APPLICATIONS of GAS CHROMATOGRAPHY
A Thesis entitled "APPLICATIONS OF GAS CHROMATOGRAPHY - MASS SPECTROMETRY IN STEROID CHEMISTRY" Submitted in part fulfilment of the requirements for admittance to the degree of Doctor of Philosophy in The University of Glasgow by T.A. Baillie, B.Sc. University of Glasgow 1973. ProQuest Number: 11017930 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 11017930 Published by ProQuest LLC(2018). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 ACKNOWLEDGEMENTS I would like to express my sincere thanks to Dr. C.3.W. Brooks for his guidance and encouragement at all times, and to Professors R.A. Raphael, F.R.S., and G.W. Kirby, for the opportunity to carry out this research. Thanks are also due to my many colleagues for useful discussions, and in particular to Dr. B.S. Middleditch who was associated with me in the work described in Section 3 of this thesis. The work was carried out during the tenure of an S.R.C. Research Studentship, which is gratefully acknowledged. Finally, I would like to thank Miss 3.H. -

AOD Biennial Review
Introduction The Drug-Free Schools and Communities Act and Part 86 of the Education Department General Administrative Regulations (EDGAR) require each institution of higher education participating in Title IV student financial assistance to certify that it has developed and implemented a program to prevent the unlawful possession, use, and distribution of drugs and alcohol on campus and at institution-recognized events and activities. Annually, the institution must distribute to current students and employees written information about its drug and alcohol abuse prevention program, including information about related sanctions. The distribution plan must include provisions for sharing this information with new students and employees who join the institution at later points in the year as well. In addition, on a biennial basis, the institution must conduct a review to determine the effectiveness of its programs and prepare a report of the review’s findings. The report must be retained and made available to the US Department of Education upon request. Notre Dame of Maryland University (“NDMU” or “the University”) acknowledges these responsibilities, including the timely review of and reporting on the effectiveness of its prevention programs. NDMU is a private, Catholic university established in 1895 with the mission to educate leaders to transform the world. NDMU is a comprehensive university that is home to Maryland's only women's college and offers a wide variety of full and part-time undergraduate, graduate, doctoral, and certificate programs to women and men. The University enrolls approximately 2300 students and has Schools of Arts, Sciences, and Business; Education; Nursing; and Pharmacy. Additionally, the University offers programs for adult students fully online and at several locations throughout the State. -

Quantification of Hair Corticosterone, DHEA and Testosterone As
animals Article Quantification of Hair Corticosterone, DHEA and Testosterone as a Potential Tool for Welfare Assessment in Male Laboratory Mice Alberto Elmi 1 , Viola Galligioni 2, Nadia Govoni 1 , Martina Bertocchi 1 , Camilla Aniballi 1 , Maria Laura Bacci 1 , José M. Sánchez-Morgado 2 and Domenico Ventrella 1,* 1 Department of Veterinary Medical Sciences, University of Bologna, 40064 Ozzano dell’Emilia, BO, Italy; [email protected] (A.E.); [email protected] (N.G.); [email protected] (M.B.); [email protected] (C.A.); [email protected] (M.L.B.) 2 Comparative Medicine Unit, Trinity College Dublin, D02 Dublin, Ireland; [email protected] (V.G.); [email protected] (J.M.S.-M.) * Correspondence: [email protected]; Tel.: +39-051-2097-926 Received: 11 November 2020; Accepted: 14 December 2020; Published: 16 December 2020 Simple Summary: Mice is the most used species in the biomedical research laboratory setting. Scientists are constantly striving to find new tools to assess their welfare, in order to ameliorate husbandry conditions, leading to a better life and scientific data. Steroid hormones can provide information regarding different behavioral tracts of laboratory animals but their quantification often require stressful sampling procedures. Hair represents a good, less invasive, alternative in such scenario and is also indicative of longer timespan due to hormones’ accumulation. The aim of the work was to quantify steroid hormones in the hair of male laboratory mice and to look for differences imputable to age and housing conditions (pairs VS groups). Age influenced all analysed hormones by increasing testosterone and dehydroepiandrosterone (DHEA) levels and decreasing corticosterone. -

Alteration of the Steroidogenesis in Boys with Autism Spectrum Disorders
Janšáková et al. Translational Psychiatry (2020) 10:340 https://doi.org/10.1038/s41398-020-01017-8 Translational Psychiatry ARTICLE Open Access Alteration of the steroidogenesis in boys with autism spectrum disorders Katarína Janšáková 1, Martin Hill 2,DianaČelárová1,HanaCelušáková1,GabrielaRepiská1,MarieBičíková2, Ludmila Máčová2 and Daniela Ostatníková1 Abstract The etiology of autism spectrum disorders (ASD) remains unknown, but associations between prenatal hormonal changes and ASD risk were found. The consequences of these changes on the steroidogenesis during a postnatal development are not yet well known. The aim of this study was to analyze the steroid metabolic pathway in prepubertal ASD and neurotypical boys. Plasma samples were collected from 62 prepubertal ASD boys and 24 age and sex-matched controls (CTRL). Eighty-two biomarkers of steroidogenesis were detected using gas-chromatography tandem-mass spectrometry. We observed changes across the whole alternative backdoor pathway of androgens synthesis toward lower level in ASD group. Our data indicate suppressed production of pregnenolone sulfate at augmented activities of CYP17A1 and SULT2A1 and reduced HSD3B2 activity in ASD group which is partly consistent with the results reported in older children, in whom the adrenal zona reticularis significantly influences the steroid levels. Furthermore, we detected the suppressed activity of CYP7B1 enzyme readily metabolizing the precursors of sex hormones on one hand but increased anti-glucocorticoid effect of 7α-hydroxy-DHEA via competition with cortisone for HSD11B1 on the other. The multivariate model found significant correlations between behavioral indices and circulating steroids. From dependent variables, the best correlation was found for the social interaction (28.5%). Observed changes give a space for their utilization as biomarkers while reveal the etiopathogenesis of ASD. -

Appendix: Common Steroid Identification—GC-MS Mass Spectra
Appendix: Common Steroid Identification—GC-MS Mass Spectra Gary Woodward, Francis Lam, and Gill Rumsby As described in Chap. 3, steroid profiling in the context of steroidogenic conditions is performed by GC-MS analysis following conjugate hydrolysis and derivatisation. Below are the mass spectra used for identification of urine steroids described throughout this book, and compiled during routine practice within our laboratory. Steroids are given in approximate order of their retention times. Androstanediol (internal standard) % 100 129 256 346 107 241 331 436 215 287 372 484 516 569 637 677 0 100 150 200 250 300 350 400 450 500 550 600 650 70 Pregnadienol % 129 50 243 267 372 173 357 0 421 494 525 556 620 665 7 100 150 200 250 300 350 400 450 500 550 600 650 70 G. Woodward · G. Rumsby Department of Clinical Biochemistry, University College London Hospitals, London, UK F. Lam Level 2-Chemistry, Health Services Laboratories, London, UK © Springer International Publishing AG, part of Springer Nature 2019 177 G. Rumsby, G. M. Woodward (eds.), Disorders of Steroidogenesis, https://doi.org/10.1007/978-3-319-96364-8 178 Appendix: Common Steroid Identification—GC-MS Mass Spectra Androsterone % 100 270 360 107 147 213 253 362 0 434 461 546 610 687 100 150 200 250 300 350 400 450 500 550 600 650 700 Aetiocholanolone % 100 270 360 105 131 213 422 0 255 362 460 506 564 644 679 100 150 200 250 300 350 400 450 500 550 600 650 700 Dehydroepiandrosterone % 100 129 268 260 358 105 374 0 211 432 459 523 592 642 682 7 100 150 200 250 300 350 400 450 500 -

A Guide to Understanding the Steroid Pathway: New Insights and Diagnostic Implications
Clinical Biochemistry 47 (2014) 5–15 Contents lists available at ScienceDirect Clinical Biochemistry journal homepage: www.elsevier.com/locate/clinbiochem A guide to understanding the steroid pathway: New insights and diagnostic implications Ronda F. Greaves a,b,⁎,GaneshJevalikarc, Jacqueline K. Hewitt b,d,e, Margaret R. Zacharin b,d,e a School of Medical Sciences, RMIT University, Victoria, Australia b Murdoch Children's Research Institute, Melbourne, Australia c Medanta Medicity Hospital, Gurgaon Haryana, India d Department of Endocrinology & Diabetes, The Royal Children's Hospital, Victoria, Australia e Department of Paediatrics, University of Melbourne, Victoria, Australia article info abstract Article history: Steroid analysis has always been complicated requiring a clear understanding of both the clinical and analytical Received 3 May 2014 aspects in order to accurately interpret results. The literature relating to this specialised area spans many decades Received in revised form 17 July 2014 and the intricacies of the steroid pathway have evolved with time. A number of key changes, including discovery Accepted 19 July 2014 of the alternative androgen pathway, have occurred in the last decade, potentially changing our understanding Available online 31 July 2014 and approach to investigating disorders of sexual development. Such investigation usually occurs in specialised paediatric centres and although preterm infants represent only a small percentage of the patient population, Keywords: Steroid pathways consideration of the persistence of the foetal adrenal zone is an additional important consideration when under- Alternative steroid pathway taking steroid hormone investigations. The recent expanded role of mass spectrometry and molecular diagnostic Tissue specific steroid metabolism methods provides significant improvements for accurate steroid quantification and identification of enzyme Steroid methods deficiencies.