Code System Representation in Phin Vads
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
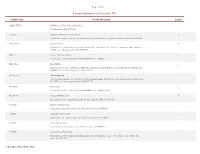
Layout Summary of Geocode File
Page 1 of 6 Layout Summary of Geocode File Variable Name Variable Description Length ADDR_TYPE USPS Record, Type of Record Matched 1 This data comes from HUD file. APT_NO Apartment Number (Address Line 2) 8 Contains the Apartment number (secondary address) portion of the street address. This data comes from HUD file. APT_TYPE Apartment Type 4 Contains the secondary address type abbreviation (Apt = Apartment, Ste = Suite, # = Apartment, RM = Room, FL = Floor, etc..). This data comes from HUD file. BG2K Census 2000 Block Group 1 It is the first character of the block ID (BLOCK2K) in the HUD file. BLK_FLG Block ID flag 1 This flag value identifies if HUD and NHIS block ID disagree. BLOCK2K is the block ID from the HUD file and COMBBLK is the block ID from the NHIS UCF file. BLKG_FLG Block group flag 1 This flag value identifies if HUD and NHIS block group disagree. BG2K is the block group from the HUD file and BLKGRP is the block group from the NHIS UCF file. BLKGRP Block Group 1 It is the first character of the block ID (COMBBLK) in the NHIS UCF file. BLOCK2K Census 2000 Block ID 4 First character is the census block group, it comes from the HUD geocoded file. C1PARC Apartment Return Code 1 Postal matcher apartment return codes. This data comes from HUD file. C1PDRC Directional Return Code 1 Postal matcher directional return codes. This data comes from HUD file. C1PGRC General Return Code 1 Postal matcher general return codes. This data comes from HUD file. C1PPRB Address Match Probability 1 Postal matcher address match probability return codes. -

Fips Pub 55-3
U.S. DEPARTMENT OF COMMERCE Technology Administration National Institute of Standards and Technology FIPS PUB 55-3 FEDERAL INFORMATION PROCESSING STANDARDS PUBLICATION (Supersedes FIPS PUB 55-2—1987 February 3 and 55DC-4—1987 January 16) GUIDELINE: CODES FOR NAMED POPULATED PLACES, PRIMARY COUNTY DIVISIONS, AND OTHER LOCATIONAL ENTITIES OF THE UNITED STATES, PUERTO RICO, AND THE OUTLYING AREAS Category: Data Standards and Guidelines Subcategory: Representation and Codes 1994 DECEMBER 28 55-3 PUB FIPS 468 . A8A3 NO.55-3 1994 FIPS PUB 55-3 FEDERAL INFORMATION PROCESSING STANDARDS PUBLICATION (Supersedes FIPS PUB 55-2—1987 February 3 and 55DC-4—1987 January 16) GUIDELINE: CODES FOR NAMED POPULATED PLACES, PRIMARY COUNTY DIVISIONS, AND OTHER LOCATIONAL ENTITIES OF THE UNITED STATES, PUERTO RICO, AND THE OUTLYING AREAS Category: Data Standards and Guidelines Subcategory: Representations and Codes Computer Systems Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899-0001 Issued December 28, 1994 U.S. Department of Commerce Ronald H. Brown, Secretary Technology Administration Mary L. Good, Under Secretary for Technology National Institute of Standards and Technology Arati Prabhakar, Director Foreword The Federal Information Processing Standards Publication Series of the National Institute of Standards and Technology (NIST) is the official publication relating to standards and guidelines adopted and promulgated under the provisions of Section 111 (d) of the Federal Property and Administrative Services Act of 1949 as amended by the Computer Security Act of 1987, Public Law 100-235. These mandates have given the Secretary of Commerce and NIST important responsibilities for improving the utilization and management of computer and related telecommunications systems in the Federal Government. -

Census of Population and Housing, 1980: Summary Tape File 1F, School Districts
DOCUMENT RESUME ED 313 413 TM 014 179 TITLE Census of Population and Housing, 1980: Summary Tape File 1F, School Districts. Technical Documentation. INSTITUTION Bureau of the Census (DOC), Washington, D.C. Data User Services Div. REPORT NO EF-000082 PUB DATE 82 NOTE 207p.; For the corresponding data file, see TM 014 180. The questionnaire is sometimes illegible due to small and blurred print. PUB TYPE Guides Non-C1-Issroom Use (055) Statistical Data (110) Tests/Evaluation Instruments (160) EDRS PRICE MF01/PC09 Plus Postage. DESCRIPTORS Assessed Valuation; *Census Figures; Databases; *Demography; Elementary Secondary Education; Family Characteristics; Geographic Regions; Hispanic Americans; *Housing; Marital Status; Online Systems; Questionnaires; Racial Distribution; *School Districts; *Statistical Surveys IDENTIFIERS *Census of Population Housing 1980 Sum Tape File 1F ABSTRACT This report provides technical documentation associated with a 1980 Census of Population and Housing Summary Tape File 1F--the School Districts File. The file contains complete-count data of population and housing aggregated by school district. Population items tabulated include age, race (provisional data), sex, marital status, Spanish origin (provisional data), household type, and household relationship. Housing items tabulated include occupancy/vacancy status, tenure, contract rent, value, condominium status, number of rooms, and plumbing facilities. Selected aggregates, means, and medians are alsc provided. Data are presented in E9 tables. Summaries are provided at the follmolng levels: national, regional, state, county, standard metropolitan area and other standard statistical geographical area, congressional district, census division, and minor civil division. Information specific to Summary Tape File 1 and information applicable to Summary Tape Files 1 through 5 are provided. -

November 5, 2003 – Letter to the Secretary – Comments on CHI
November 5, 2003 The Honorable Tommy G. Thompson Secretary Department of Health and Human Services 200 Independence Avenue SW Washington, D.C. 20201 Dear Secretary Thompson: The National Committee on Vital and Health Statistics (NCVHS) commends you for your commitment toward government wide adoption of clinical data standards that you first announced on March 21, 2003. NCVHS recognizes and appreciates that there is new momentum to adopt clinical data standards that is driven by you and the Consolidated Healthcare Informatics Initiative (CHI). Consequently, NCVHS is now working closely with CHI to study, select and recommend domain specific patient medical record information (PMRI) terminology standards. We have mutually developed a process that allows NCVHS to discuss in open, interactive sessions CHI recommendations as part of the CHI Council acceptance process. The NCVHS has the following comments on the attached set of CHI domain area recommendations: • The NCVHS concurs with the CHI recommendations for Interventions and Procedures: Part B, Laboratory Test Order Names. • The NCVHS concurs with the CHI recommendations for the Medication Domain as modified in the attached document. We further note: o the need for additional funding for the Food and Drug Administration (FDA) to expedite the publication of a publicly available version of their Unique Ingredient Identifier (UNII) codes and to provide continued funding to maintain the UNII code standard; o that the dosage and administration sub-domain be investigated in the next phase of the CHI process; and o that the FDA National Drug Code (NDC) process be investigated and improvements identified be expeditiously pursued. • The NCVHS concurs with the CHI recommendations for the Immunization Domain as modified in the attached document. -

Ata Lements for Mergency Epartment Ystems
D ata E lements for E mergency D epartment S ystems Release 1.0 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES CDCCENTERS FOR DISEASE CONTROL AND PREVENTION D ata E lements for E mergency D epartment S ystems Release 1.0 National Center for Injury Prevention and Control Atlanta, Georgia 1997 Data Elements for Emergency Department Systems, Release 1.0 (DEEDS) is the result of contributions by participants in the National Workshop on Emergency Department Data, held January 23-25, 1996, in Atlanta, Georgia, subsequent review and comment by individuals who read Release 1.0 in draft form, and finalization by a multidisciplinary writing committee. DEEDS is a set of recommendations published by the National Center for Injury Prevention and Control of the Centers for Disease Control and Prevention: Centers for Disease Control and Prevention David Satcher, MD, PhD, Director National Center for Injury Prevention and Control Mark L. Rosenberg, MD, MPP, Director Division of Acute Care, Rehabilitation Research, and Disability Prevention Richard J. Waxweiler, PhD, Director Acute Care Team Daniel A. Pollock, MD, Leader Production services were provided by the staff of the Office of Communication Resources, National Center for Injury Prevention and Control, and the Management Analysis and Services Office: Editing Valerie R. Johnson Cover Design and Layout Barbara B. Lord Use of trade names is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services. This document and subsequent revisions can be found at the National Center for Injury Prevention and Control Web site: http://www.cdc.gov/ncipc/pub-res/deedspage.htm Suggested Citation: National Center for Injury Prevention and Control. -

(USCDI), Version 1, July 2020 Errata
Allergies and Intolerances .... 5 The USCDI is a standardized Assessment and Plan of set of health data classes and Treatment ............................ 5 Care Team Member(s) ......... 6 constituent data elements for Clinical Notes ........................ 7 nationwide, interoperable Goals ..................................... 8 Health Concerns ................... 8 health information exchange. Immunizations ...................... 9 Laboratory ............................ 9 A USCDI “Data Class” is an aggregation of various Data Elements by a common theme or use case. Medications ........................ 10 Patient Demographics ........ 10 A USCDI “Data Element” is the most granular level Problems ............................ 12 at which a piece of data is represented in the USCDI Procedures ......................... 12 for exchange. Provenance ........................ 13 Smoking Status ................... 13 Unique Device Identifier(s) for a Patient’s Implantable Device(s) ............................. 13 Vital Signs ........................... 14 2 Version History Version # Description of change Version Date 1 First Publication February 2020 1 (July 2020 Corrections to Applicable Standards July 2020 Errata) • Substance (Medication) - Removed UCUM • Patient’s Goals, Health Concerns, Assessment and Plan of Treatment - Removed LOINC, SNOMED CT • Laboratory Tests, Values/Results - Removed SNOMED CT and UCUM • Medications - Removed UCUM 3 USCDI v1 Summary of Data Classes and Data Elements Allergies and Intolerances Laboratory Smoking Status -

Report-Health Terminologies and Vocabularies Environmental Scan
Health Terminologies and Vocabularies Environmental Scan Conducted for the National Committee on Vital and Health Statistics Subcommittee on Standards September 14, 2018 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Health Terminologies and Vocabularies Environmental Scan NCVHS Subcommittee on Standards This report was written by Susan L. Roy, MS, MLS, SNOMED CT Coordinator, and Vivian A. Auld, MLIS, Senior Specialist for Health Data Standards, both with the National Institutes of Health (NIH), National Library of Medicine, in collaboration with the NCVHS Standards Subcommittee: NCVHS Membership William W. Stead, MD, NCVHS Chair Nicholas L. Coussoule,* Subcommittee Co-chair Alexandra Goss,* Subcommittee Co-chair Bruce B. Cohen, PhD David A. Ross, ScD Debra Strickland, MS* Denise E. Love, BSN, MBA* Jacki Monson, JD Linda L. Kloss, MA, RHIA*, Terminology and Vocabulary Project Lead Llewellyn J. Cornelius, PhD Richard W. Landen, MPH, MBA* Robert L. Phillips, Jr., MD, MSPH Roland J. Thorpe, Jr., PhD Vickie M. Mays, PhD, MSPH *Member of the Subcommittee on Standards Rebecca Hines, MHS, NCVHS Executive Secretary/DFO Health Scientist National Center for Health Statistics, CDC, HHS Rashida Dorsey, PhD, MPH, NCVHS Executive Staff Director Director, Division of Data Policy Senior Advisor on Minority Health and Health Disparities Office of Science and Data Policy Office of the Assistant Secretary for Planning and Evaluation, HHS The National Committee on Vital and Health Statistics (NCVHS) serves as the advisory committee to the Secretary of Health and Human Services (HHS) on health data, statistics, privacy, national health information policy, and the Health Insurance Portability and Accountability Act (HIPAA) (42U.S.C.242k[k]). -

(Child Core Set) Technical Specifications and Reso
Core Set of Children's Health Care Quality Measures for Medicaid and CHIP (Child Core Set) Technical Specifications and Resource Manual for Federal Fiscal Year 2019 Reporting February 2019 Center for Medicaid and CHIP Services Centers for Medicare & Medicaid Services This page left blank for double-sided copying ii ACKNOWLEDGMENTS For Proprietary Codes: CPT® codes copyright 2018 American Medical Association. All rights reserved. CPT is a trademark of the American Medical Association. No fee schedules, basic units, relative values or related listings are included in CPT. The AMA assumes no liability for the data contained herein. Applicable FARS/DFARS restrictions apply to government use. SNOMED CLINICAL TERMS (SNOMED CT®) copyright 2004-2018 The International Health Terminology Standards Development Organisation. All Rights Reserved. The International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) is published by the World Health Organization (WHO). ICD-9-CM is an official Health Insurance Portability and Accountability Act standard. The International Classification of Diseases, 9th Revision, Procedure Coding System (ICD-9-PCS) is published by the World Health Organization (WHO). ICD-9-PCS is an official Health Insurance Portability and Accountability Act standard. The International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) is published by the World Health Organization (WHO). ICD-10-CM is an official Health Insurance Portability and Accountability Act standard. The International Classification of Diseases, 10th Revision, Procedure Coding System (ICD-10-PCS) is published by the World Health Organization (WHO). ICD-10-PCS is an official Health Insurance Portability and Accountability Act standard. This material contains content from LOINC® (http://loinc.org). -

Billing and Coding Considerations for BLINCYTO®
BLINCYTO® Billing Information Sheet Billing and Coding Considerations for BLINCYTO® This Information Sheet is intended to help healthcare professionals understand the key billing and coding considerations for BLINCYTO® and its related services and supplies when using the FDA-approved dosing options in inpatient and outpatient treatment settings. Updates for 2018 include: • Information on billing the 7-day infusion option (7-DIO) in addition to the shorter-duration 24-hour and 48-hour bags BLINCYTO® Dosing Options1 Dose per vial X number of Dosing option Number of billing units single-dose vials (SDVs*) 24-hour 35 mcg X 1 vial 35 48-hour 35 mcg X 1-2 vials 35-70 7-day 35 mcg X 4-6 vials 140-210 • New coding requirement for BLINCYTO® administered in the outpatient setting of 340B hospitals – The Centers for Medicare & Medicaid Services (CMS) requires use of new modifiers on Medicare claims for drugs acquired under the 340B Drug Discount Program2 • Medicare patients receiving BLINCYTO® in the inpatient setting are no longer eligible for additional reimbursement3 – The new technology add-on payment (NTAP) designation has been retired; hospitals may still be eligible for outlier payments based on payer guidance and managed care contract provisions *Number of SDVs depends on dose, infusion duration, and patient's weight.1 Please note that the information in this resource is intended to be educational and is not a guarantee of reimbursement. Coverage, coding, and billing requirements vary by health plan so be sure to check with individual payers for detailed guidance. Indications BLINCYTO® is indicated for the treatment of B-cell precursor acute lymphoblastic leukemia (ALL) in first or second complete remission with minimal residual disease (MRD) greater than or equal to 0.1% in adults and children. -

The Role of Family Practice in Different Health Care Systems
January 2002 · Vol. 51, No. 1 JFP ONLINE The Role of Family Practice in Different Health Care Systems A Comparison of Reasons for Encounter, Diagnoses, and Interventions in Primary Care Populations in the Netherlands, Japan, Poland, and the United States I.M. Okkes, MA, PhD; G.O. Polderman, MD; G.E. Fryer, PhD; T. Yamada, MD; M. Bujak, MD; S.K. Oskam, MSc, PhD; L.A. Green, MD; H. Lamberts, MD, PhD Amsterdam and Amstelveen, the Netherlands; Tokyo, Japan; Katowice, Poland; and Washington, DC Submitted, revised, October 21, 2001. From the Academic Medical Center/University of Amsterdam, Division of Public Health, Department of Family Practice, Amsterdam (I.M.O., S.K.O., H.L.); Family Physician, Transition Project, Amstelveen (G.O.P.); the Robert Graham Center: Policy Studies in Family Practice and Primary Care, Washington, DC (G.E.F., L.A.G.); the Japanese Association for Development of Community Medicine, Tokyo (T.Y.); and the Silesian Medical School, Katowice (M.B.). Reprint requests should be addressed to I.M. Okkes, MA, PhD, Academic Medical Center/University of Amsterdam, Division Public Health, Department of Family Practice, Meibergdreef 15, 1105 AZ Amsterdam, the Netherlands. E-mail: [email protected]. z OBJECTIVE: Our goal was to compare the content of family practice in different countries using databases containing information on reasons for encounter, diagnoses, and interventions that are coded with or can be addressed by the International Classification of Primary Care (ICPC). z STUDY DESIGN: In the Netherlands, Japan, and Poland data were collected identically with an electronic patient record (Transhis). -

2018 Annual Surveillance Report of Drug-Related Risks and Outcomes
2018 ANNUAL SURVEILLANCE REPORT OF DRUG-RELATED RISKS AND OUTCOMES UNITED STATES Centers for Disease Control and Prevention National Center for Injury Prevention and Control Annual Surveillance Report of Drug-Related Risks and Outcomes | United States CDC National Center for Injury Prevention and Control | 2018 ACKNOWLEDGEMENTS This Surveillance Report was prepared by staff from the National Disclaimer Center for Injury Prevention and Control (NCIPC), Centers for Disease The findings and conclusions in Control and Prevention (CDC), U.S. Department of Health and Human this report are those of the authors Services, Atlanta, Georgia. and do not necessarily represent the views of the Centers for Disease Control and Prevention. This Contributors to this report included: Brooke E. Hoots, PhD, MSPH1, 2 3 research was supported in part by Likang Xu, MD, MS , Mbabazi Kariisa, PhD , Nana Otoo Wilson, PhD, an appointment to the Research 1 1 1,4 MPH, MSc , Rose A. Rudd, MSPH , Lawrence Scholl, PhD, MPH , Lyna Participation Program at the Centers 1 1 Schieber, MD, DPhil , and Puja Seth, PhD for Disease Control and Prevention 1Division of Unintentional Injury Prevention (DUIP), National Center for administered by the Oak Ridge Injury Prevention and Control, CDC Institute for Science and Education 2Division of Analysis, Research, and Practice Integration (DARPI), through an interagency agreement National Center for Injury Prevention and Control, CDC between the U.S. Department of 3Oak Ridge Institute for Science and Education, Oak Ridge, TN Energy and CDC. 4Epidemic Intelligence Service, CDC All material in this report is in the public domain and may be used Corresponding authors: Brooke E. -

보건의료정보 표준화 연구 Research of Health Information Standardization
보안과제( ), 일반과제( o ) A040165 보건의료기술연구개발(의료정보기술개발) 보건의료정보 표준화 연구 Research of Health Information Standardization 한국보건산업진흥원 한국보건산업진흥원 제 출 문 보건복지부 장관 귀하 이 보고서를 “보건의료정보 표준화 연구(고유번호 : 0412-MI02-0416-0001)” 과제의 최종보고서로 제출합니다. 2005 . 6 . 30 주관연구기관 : 한국보건산업진흥원 직인 주관연구책임자 : 공 재 근 직인 ❀ 보건의료정보 표준화에 참여해 주신 분들 ❀ 표준화위원회 •위원장 : 김 윤(서울의대 의료관리학교실) •표준화위원 서창해(인하의대 방사선과) 이영종(경원대 한의대) 감 신(경북의대 예방의학교실) 최행정(국민건강보험공단 일산병원 의무기록팀) 박현애(서울대 간호대) 김석일(가톨릭의대 예방의학교실) 김옥남(서울아산중앙병원 의무기록팀) 신형식(라이프엠) 황 건(인하대병원 성형외과) 이태한(보건복지부 혁신인사기획관) 이갑노(고려대 진단검사의학과) 임종규(보건복지부 보건의료서비스산업육성팀) 이숙향(숙명여대 임상약학대학원) 선우종성(보건복지부 정보화담당관실) 김보연(건강보험심사평가원 급여관리실) 박민수(보건복지부 공공보건정책과) 자문위원회 •위원장 : 곽연식 •자문위원 채영문(연세대 보건대학원) 윤순녕(서울대 간호대) 김명기(서울대 치과의료관리학과) 신현택(숙명여대 약학대학) 지제근(서울의대 병리과) 염용권(한국보건산업진흥원 보건의료기술연구 사업관리본부) 기획위원회 •위원장 : 김 윤(서울의대 의료관리학교실) •기획위원 박현애(서울대 간호대) 김곤희(보건복지부 공공보건정책과) 김석일(가톨릭의대 예방의학교실) 좌용권(한국보건산업진흥원) 신형식(라이프엠) 부유경(인하대병원 의료정보과) 김소윤(보건복지부 보건의료서비스산업육성팀) 박경모(경희대 동서의료공학과) 기획위원회 지원 이관익(한국보건산업진흥원) 곽미숙(서울대의대 의료관리학교실) 박정선(한국보건산업진흥원) 손수인(한국보건산업진흥원) 구현경(서울대의대 의료관리학교실) - 3 - 의료용어분과위원회 •분과위원장 : 서창해(인하대 의대 방사선과학) •분과위원 부유경(인하대병원 의료정보과) 김대성(한진정보통신) 최선근(인하의대 외과) 심진수(한진정보통신) 이연숙(인하대병원 전산정보팀) •자문위원 진호준(서울대 분당병원 내과) 김지영(분당재생병원 의무기록과) 최진욱(서울대 의용공학과) 유연순(이대목동병원 의무기록과) 박찬병(수원의료원) 서진숙(삼성서울병원 의무기록파트) 박덕영(강릉대학교 예방치학교실) 강재은(서울대 분당병원 의무기록파트) 서순원(단국대병원 의무기록과) 이은미(신촌세브란스병원 의무기록과) 최행정(건강보험공단일산병원 의무기록팀) 김란혜(경희의료원 의무기록과) 김옥남(서울아산병원 의무기록팀) 보건용어분과위원회 •분과위원장 : 감신(경북대의대 예방의학교실) •분과위원 이은미(신촌세브란스병원 의무기록과) 진대구(대구 성서병원) 이중정(계명대학교 의과대학) 정은경(질병관리본부) 이원영(중앙대학교 의과대학) 이상원(대구카톨릭대학교 의과대학) 황태윤(영남대학교 의과대학) 김찬호(경기도 안양시 보건소) 이인숙(서울대학교 간호대학)