(Child Core Set) Technical Specifications and Reso
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MHSUDS Information Notice No.: 16-051
State of California—Health and Human Services Agency Department of Health Care Services JENNIFER KENT EDMUND G. BROWN JR. DIRECTOR GOVERNOR DATE: October 7, 2016 MHSUDS INFORMATION NOTICE N O.: 16-051 TO: COUNTY BEHAVIORAL HEALTH DIRECTORS COUNTY BEHAVIORAL HEALTH DIRECTORS ASSOCIATION OF CALIFORNIA CALIFORNIA COUNCIL OF COMMUNITY BEHAVIORAL HEALTH AGENCIES CALIFORNIA ALLIANCE OF CHILD AND FAMILY SERVICES SUBJECT: IMPLEMENTATION OF THE DIAGNOSTIC AND STATISTICAL MANUAL OF MENTAL DISORDERS (DSM-5), FIFTH EDITION REFERENCES: MHSD INFORMATION NOTICE 13-22 MHSUDS INFORMATION NOTICE 14-040 MHSUDS INFORMATION NOTICE 15-003 MHSUDS INFORMATION NOTICE 15-030 PURPOSE The purpose of this Information Notice is to direct Mental Health Plans (MHPs) to use the American Psychiatric Association (APA) DS M-5 to make diagnostic determinations for the purposes of determining if beneficiaries meet medical necessity criteria for Medi-Cal specialty mental health services (SMHS). This Information Notice updates information previously provided in Mental Health Services Division (MHSD) Information Notice 13-22. BACKGROUND The APA issued the DSM-5 on May 20, 2013. The DSM-5 provides diagnostic criteria and codes as well as corresponding International Classification of Diseases-10 (ICD-10) codes. Since March 2013, the Department of Health Care Services (DHCS) has collaborated with internal and external stakeholders and partners to address implementation issues specific to SMHS related to the DSM-5. Mental Health & Substance Use Disorder Services 1501 Capitol Avenue, MS 4000, P.O. Box 997413 Sacramento, CA 95899-7413 Phone: (916) 440-7800 Fax: (916) 319-8219 Internet Address: www.dhcs.ca.gov MHSUDS INFORMATION NOTICE N O.: 16-051 October 7, 2016 Page 2 DHCS has issued previous MHSUDS Information Notices1 that instruct MHPs to report only ICD-10 codes for claiming and diagnoses reporting purposes and provide ICD-10 procedural and diagnosis crosswalk documents f or SMHS. -

ICD-10 International Statistical Classification of Diseases and Related Health Problems
ICD-10 International Statistical Classification of Diseases and Related Health Problems 10th Revision Volume 2 Instruction manual 2010 Edition WHO Library Cataloguing-in-Publication Data International statistical classification of diseases and related health problems. - 10th revision, edition 2010. 3 v. Contents: v. 1. Tabular list – v. 2. Instruction manual – v. 3. Alphabetical index. 1.Diseases - classification. 2.Classification. 3.Manuals. I.World Health Organization. II.ICD-10. ISBN 978 92 4 154834 2 (NLM classification: WB 15) © World Health Organization 2011 All rights reserved. Publications of the World Health Organization are available on the WHO web site (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press through the WHO web site (http://www.who.int/about/licensing/copyright_form). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. -

Clinical Vocabularies for Global Real World Evidence (RWE) | Evidera
Clinical Vocabularies for Global RWE Analysis Don O’Hara, MS Senior Research Associate, Real-World Evidence, Evidera Vernon F. Schabert, PhD Senior Research Scientist, Real-World Evidence, Evidera Introduction significant volume of real-world evidence (RWE) analyses continue to be conducted with data A repurposed from healthcare administrative Don O’Hara Vernon F. Schabert databases. The range of sources represented by those databases has grown in response to demand for richer description of patient health status and outcomes. information for improved quality and coordination Data availability, including the range of available data of care, often using standardized messaging systems sources, has grown unevenly across the globe in response such as Health Level 7 (HL7). These messages are to country-specific market and regulatory dynamics. only as good as the standardization of codes between Nonetheless, as demand globalizes for RWE insights from message senders and receivers, which motivates the databases, those demands increase pressure on analysts encoding of facts using common code sets. The second to find ways to bridge differences between local data is the increased availability of common data models to sources to achieve comparable insights across regions. standardize the extraction and analysis of these data for RWE and drug safety purposes. While common data One of the challenges in bridging differences across models make compromises on the structure of tables databases is the codes used to represent key clinical and fields extracted from healthcare systems such as facts. Historically, RWE database studies have leveraged electronic medical records (EMR) and billing systems, local code sets for cost-bearing healthcare services they can improve consistency and replicability of analyses such as drugs, procedures, and laboratory tests. -
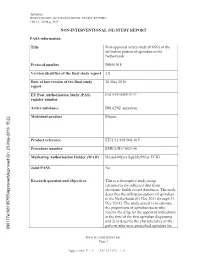
Non-Interventional (Ni) Study Report
Apixaban B0661018 NON-INTERVENTIONAL STUDY REPORT FINAL, 20 May 2015 NON-INTERVENTIONAL (NI) STUDY REPORT PASS information Title Post-approval safety study (PASS) of the utilization pattern of apixaban in the Netherlands Protocol number B0661018 Version identifier of the final study report 1.0 Date of last version of the final study 20 May 2016 report EU Post Authorisation Study (PAS) ENCEPP/SDPP/5177 register number Active substance B01AF02 apixaban Medicinal product Eliquis -2016 15:22 y Product reference EU/1/11/691/001-015 Procedure number EMEA/H/C/002148 Marketing Authorisation Holder (MAH) Bristol-Myers Squibb/Pfizer EEIG roved On: 23-Ma Joint PASS No pp Research question and objectives This is a descriptive study using roved\A retrospectively collected data from pp electronic health record databases. The study describes the utilization pattern of apixaban in the Netherlands (01 Dec 2011 through 31 Dec 2014). The study aimed 1) to estimate the proportions of apixaban users who receive the drug for the approved indications at the time of the first apixaban dispensing and 2) to describe the characteristics of the patients who were prescribed apixaban for 090177e188105755\A PFIZER CONFIDENTIAL Page 1 Approved v 1.0 930102376 1.0 Apixaban B0661018 NON-INTERVENTIONAL STUDY REPORT FINAL, 20 May 2015 on-label and off-label indications. Country(-ies) of study The Netherlands Author Marketing Authorisation Holder(s) Marketing Authorisation Holder(s) Bristol-Myers Squibb/Pfizer EEIG -2016 15:22 y MAH contact person roved On: 23-Ma pp roved\A pp This document contains confidential information belonging to Pfizer. -

November 5, 2003 – Letter to the Secretary – Comments on CHI
November 5, 2003 The Honorable Tommy G. Thompson Secretary Department of Health and Human Services 200 Independence Avenue SW Washington, D.C. 20201 Dear Secretary Thompson: The National Committee on Vital and Health Statistics (NCVHS) commends you for your commitment toward government wide adoption of clinical data standards that you first announced on March 21, 2003. NCVHS recognizes and appreciates that there is new momentum to adopt clinical data standards that is driven by you and the Consolidated Healthcare Informatics Initiative (CHI). Consequently, NCVHS is now working closely with CHI to study, select and recommend domain specific patient medical record information (PMRI) terminology standards. We have mutually developed a process that allows NCVHS to discuss in open, interactive sessions CHI recommendations as part of the CHI Council acceptance process. The NCVHS has the following comments on the attached set of CHI domain area recommendations: • The NCVHS concurs with the CHI recommendations for Interventions and Procedures: Part B, Laboratory Test Order Names. • The NCVHS concurs with the CHI recommendations for the Medication Domain as modified in the attached document. We further note: o the need for additional funding for the Food and Drug Administration (FDA) to expedite the publication of a publicly available version of their Unique Ingredient Identifier (UNII) codes and to provide continued funding to maintain the UNII code standard; o that the dosage and administration sub-domain be investigated in the next phase of the CHI process; and o that the FDA National Drug Code (NDC) process be investigated and improvements identified be expeditiously pursued. • The NCVHS concurs with the CHI recommendations for the Immunization Domain as modified in the attached document. -

Ata Lements for Mergency Epartment Ystems
D ata E lements for E mergency D epartment S ystems Release 1.0 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES CDCCENTERS FOR DISEASE CONTROL AND PREVENTION D ata E lements for E mergency D epartment S ystems Release 1.0 National Center for Injury Prevention and Control Atlanta, Georgia 1997 Data Elements for Emergency Department Systems, Release 1.0 (DEEDS) is the result of contributions by participants in the National Workshop on Emergency Department Data, held January 23-25, 1996, in Atlanta, Georgia, subsequent review and comment by individuals who read Release 1.0 in draft form, and finalization by a multidisciplinary writing committee. DEEDS is a set of recommendations published by the National Center for Injury Prevention and Control of the Centers for Disease Control and Prevention: Centers for Disease Control and Prevention David Satcher, MD, PhD, Director National Center for Injury Prevention and Control Mark L. Rosenberg, MD, MPP, Director Division of Acute Care, Rehabilitation Research, and Disability Prevention Richard J. Waxweiler, PhD, Director Acute Care Team Daniel A. Pollock, MD, Leader Production services were provided by the staff of the Office of Communication Resources, National Center for Injury Prevention and Control, and the Management Analysis and Services Office: Editing Valerie R. Johnson Cover Design and Layout Barbara B. Lord Use of trade names is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services. This document and subsequent revisions can be found at the National Center for Injury Prevention and Control Web site: http://www.cdc.gov/ncipc/pub-res/deedspage.htm Suggested Citation: National Center for Injury Prevention and Control. -

(USCDI), Version 1, July 2020 Errata
Allergies and Intolerances .... 5 The USCDI is a standardized Assessment and Plan of set of health data classes and Treatment ............................ 5 Care Team Member(s) ......... 6 constituent data elements for Clinical Notes ........................ 7 nationwide, interoperable Goals ..................................... 8 Health Concerns ................... 8 health information exchange. Immunizations ...................... 9 Laboratory ............................ 9 A USCDI “Data Class” is an aggregation of various Data Elements by a common theme or use case. Medications ........................ 10 Patient Demographics ........ 10 A USCDI “Data Element” is the most granular level Problems ............................ 12 at which a piece of data is represented in the USCDI Procedures ......................... 12 for exchange. Provenance ........................ 13 Smoking Status ................... 13 Unique Device Identifier(s) for a Patient’s Implantable Device(s) ............................. 13 Vital Signs ........................... 14 2 Version History Version # Description of change Version Date 1 First Publication February 2020 1 (July 2020 Corrections to Applicable Standards July 2020 Errata) • Substance (Medication) - Removed UCUM • Patient’s Goals, Health Concerns, Assessment and Plan of Treatment - Removed LOINC, SNOMED CT • Laboratory Tests, Values/Results - Removed SNOMED CT and UCUM • Medications - Removed UCUM 3 USCDI v1 Summary of Data Classes and Data Elements Allergies and Intolerances Laboratory Smoking Status -

Report-Health Terminologies and Vocabularies Environmental Scan
Health Terminologies and Vocabularies Environmental Scan Conducted for the National Committee on Vital and Health Statistics Subcommittee on Standards September 14, 2018 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Health Terminologies and Vocabularies Environmental Scan NCVHS Subcommittee on Standards This report was written by Susan L. Roy, MS, MLS, SNOMED CT Coordinator, and Vivian A. Auld, MLIS, Senior Specialist for Health Data Standards, both with the National Institutes of Health (NIH), National Library of Medicine, in collaboration with the NCVHS Standards Subcommittee: NCVHS Membership William W. Stead, MD, NCVHS Chair Nicholas L. Coussoule,* Subcommittee Co-chair Alexandra Goss,* Subcommittee Co-chair Bruce B. Cohen, PhD David A. Ross, ScD Debra Strickland, MS* Denise E. Love, BSN, MBA* Jacki Monson, JD Linda L. Kloss, MA, RHIA*, Terminology and Vocabulary Project Lead Llewellyn J. Cornelius, PhD Richard W. Landen, MPH, MBA* Robert L. Phillips, Jr., MD, MSPH Roland J. Thorpe, Jr., PhD Vickie M. Mays, PhD, MSPH *Member of the Subcommittee on Standards Rebecca Hines, MHS, NCVHS Executive Secretary/DFO Health Scientist National Center for Health Statistics, CDC, HHS Rashida Dorsey, PhD, MPH, NCVHS Executive Staff Director Director, Division of Data Policy Senior Advisor on Minority Health and Health Disparities Office of Science and Data Policy Office of the Assistant Secretary for Planning and Evaluation, HHS The National Committee on Vital and Health Statistics (NCVHS) serves as the advisory committee to the Secretary of Health and Human Services (HHS) on health data, statistics, privacy, national health information policy, and the Health Insurance Portability and Accountability Act (HIPAA) (42U.S.C.242k[k]). -

Inappropriate Primary Diagnosis Codes Policy, Professional
Commercial Reimbursement Policy CMS 1500 Policy Number 2020R0122C Inappropriate Primary Diagnosis Codes Policy, Professional IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY You are responsible for submission of accurate claims. This reimbursement policy is intended to ensure that you are reimbursed based on the code or codes that correctly describe the health care services provided. UnitedHealthcare reimbursement policies may use Current Procedural Terminology (CPT®*), Centers for Medicare and Medicaid Services (CMS) or other coding guidelines. References to CPT or other sources are for definitional purposes only and do not imply any right to reimbursement. This reimbursement policy applies to all health care services billed on CMS 1500 forms and, when specified, to those billed on UB04 forms. Coding methodology, industry-standard reimbursement logic, regulatory requirements, benefits design and other factors are considered in developing reimbursement policy. This information is intended to serve only as a general reference resource regarding UnitedHealthcare’s reimbursement policy for the services described and is not intended to address every aspect of a reimbursement situation. Accordingly, UnitedHealthcare may use reasonable discretion in interpreting and applying this policy to health care services provided in a particular case. Further, the policy does not address all issues related to reimbursement for health care services provided to UnitedHealthcare enrollees. Other factors affecting reimbursement may supplement, modify or, in some cases, supersede this policy. These factors may include, but are not limited to: legislative mandates, the physician or other provider contracts, the enrollee’s benefit coverage documents and/or other reimbursement, medical or drug policies. Finally, this policy may not be implemented exactly the same way on the different electronic claims processing systems used by UnitedHealthcare due to programming or other constraints; however, UnitedHealthcare strives to minimize these variations. -

G11.11: New Icd-10-Cm (Diagnosis) Code for Friedreich's Ataxia
G11.11: NEW ICD-10-CM (DIAGNOSIS) CODE FOR FRIEDREICH’S ATAXIA EFFECTIVE OCTOBER 1, 2020 CALL TO ACTION – PROVIDE THIS INFO TO YOUR CLINICIANS Starting on October 1, 2020, at your next appointments with any clinician, ask that your ICD-10 diagnosis code for FA be updated to G11.11 (previous code for FA was G11.1 > add a “1” > G11.11). Your clinician can use multiple ICD-10 diagnosis codes based on your need for medical care (e.g. G11.11 – FA, I49.9 - cardiac arrhythmia, Z13.1 – encounter for screening for diabetes mellitus). Please email [email protected] if you or your clinician has questions regarding use of G11.11 PREVIOUS CODING FOR FRIEDREICH’S ATAXIA ICD-9 system had a specific code for FA: 334.0 In conversion to ICD-10, the code for FA became less specific because it covered a group of conditions described as “early-onset cerebellar ataxias”. The previous FA ICD-10 diagnosis code was G11.1. BENEFITS OF THE NEW FA-SPECIFIC ICD-10-CM CODE G11.11 • Fewer rejected health insurance claims • Payer coverage determinations without need for direct access to patients’ medical records for review of medical necessity • Monitor adherence to clinical management guidelines and track care outcomes • Evaluate symptom progression of FA; additional ICD-10 codes can be applied to reflect context of FA diagnosis, such as for falls, surgeries, medications, and co-morbidities • Enhanced ability to track disease prevalence BACKGROUND ON ICD CODING SYSTEM ICD = International Classification of Diseases The ICD coding system was established by the World Health Organization; currently used by ~100 countries for reporting cause of death and epidemiology statistics. -

Billing and Coding Considerations for BLINCYTO®
BLINCYTO® Billing Information Sheet Billing and Coding Considerations for BLINCYTO® This Information Sheet is intended to help healthcare professionals understand the key billing and coding considerations for BLINCYTO® and its related services and supplies when using the FDA-approved dosing options in inpatient and outpatient treatment settings. Updates for 2018 include: • Information on billing the 7-day infusion option (7-DIO) in addition to the shorter-duration 24-hour and 48-hour bags BLINCYTO® Dosing Options1 Dose per vial X number of Dosing option Number of billing units single-dose vials (SDVs*) 24-hour 35 mcg X 1 vial 35 48-hour 35 mcg X 1-2 vials 35-70 7-day 35 mcg X 4-6 vials 140-210 • New coding requirement for BLINCYTO® administered in the outpatient setting of 340B hospitals – The Centers for Medicare & Medicaid Services (CMS) requires use of new modifiers on Medicare claims for drugs acquired under the 340B Drug Discount Program2 • Medicare patients receiving BLINCYTO® in the inpatient setting are no longer eligible for additional reimbursement3 – The new technology add-on payment (NTAP) designation has been retired; hospitals may still be eligible for outlier payments based on payer guidance and managed care contract provisions *Number of SDVs depends on dose, infusion duration, and patient's weight.1 Please note that the information in this resource is intended to be educational and is not a guarantee of reimbursement. Coverage, coding, and billing requirements vary by health plan so be sure to check with individual payers for detailed guidance. Indications BLINCYTO® is indicated for the treatment of B-cell precursor acute lymphoblastic leukemia (ALL) in first or second complete remission with minimal residual disease (MRD) greater than or equal to 0.1% in adults and children. -

The Role of Family Practice in Different Health Care Systems
January 2002 · Vol. 51, No. 1 JFP ONLINE The Role of Family Practice in Different Health Care Systems A Comparison of Reasons for Encounter, Diagnoses, and Interventions in Primary Care Populations in the Netherlands, Japan, Poland, and the United States I.M. Okkes, MA, PhD; G.O. Polderman, MD; G.E. Fryer, PhD; T. Yamada, MD; M. Bujak, MD; S.K. Oskam, MSc, PhD; L.A. Green, MD; H. Lamberts, MD, PhD Amsterdam and Amstelveen, the Netherlands; Tokyo, Japan; Katowice, Poland; and Washington, DC Submitted, revised, October 21, 2001. From the Academic Medical Center/University of Amsterdam, Division of Public Health, Department of Family Practice, Amsterdam (I.M.O., S.K.O., H.L.); Family Physician, Transition Project, Amstelveen (G.O.P.); the Robert Graham Center: Policy Studies in Family Practice and Primary Care, Washington, DC (G.E.F., L.A.G.); the Japanese Association for Development of Community Medicine, Tokyo (T.Y.); and the Silesian Medical School, Katowice (M.B.). Reprint requests should be addressed to I.M. Okkes, MA, PhD, Academic Medical Center/University of Amsterdam, Division Public Health, Department of Family Practice, Meibergdreef 15, 1105 AZ Amsterdam, the Netherlands. E-mail: [email protected]. z OBJECTIVE: Our goal was to compare the content of family practice in different countries using databases containing information on reasons for encounter, diagnoses, and interventions that are coded with or can be addressed by the International Classification of Primary Care (ICPC). z STUDY DESIGN: In the Netherlands, Japan, and Poland data were collected identically with an electronic patient record (Transhis).