Hair and Nail Structure and Function
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abnormal Hair Follicle Development and Altered Cell Fate of Follicular
ERRATUM Development 137, 1775 (2010) doi:10.1242/dev.053116 Abnormal hair follicle development and altered cell fate of follicular keratinocytes in transgenic mice expressing Np63 Rose-Anne Romano, Kirsten Smalley, Song Liu and Satrajit Sinha There was a technical problem with the ePress version of Development 137, 1431-1439 published on 24 March 2010. A number of Greek symbols were missing from the text and figure headings in the Results section. A replacement ePress article was published on 31 March 2010. The online issue and print copy are correct. We apologise to authors and readers for this error. DEVELOPMENT AND STEM CELLS RESEARCH ARTICLE 1431 Development 137, 1431-1439 (2010) doi:10.1242/dev.045427 © 2010. Published by The Company of Biologists Ltd Abnormal hair follicle development and altered cell fate of follicular keratinocytes in transgenic mice expressing DNp63a Rose-Anne Romano1, Kirsten Smalley1, Song Liu2,3 and Satrajit Sinha1,* SUMMARY The transcription factor p63 plays an essential role in epidermal morphogenesis. Animals lacking p63 fail to form many ectodermal organs, including the skin and hair follicles. Although the indispensable role of p63 in stratified epithelial skin development is well established, relatively little is known about this transcriptional regulator in directing hair follicle morphogenesis. Here, using specific antibodies, we have established the expression pattern of DNp63 in hair follicle development and cycling. DNp63 is expressed in the developing hair placode, whereas in mature hair its expression is restricted to the outer root sheath (ORS), matrix cells and to the stem cells of the hair follicle bulge. To investigate the role of DNp63 in hair follicle morphogenesis and cycling, we have utilized a Tet-inducible mouse model system with targeted expression of this isoform to the ORS of the hair follicle. -

Anatomy and Physiology of Hair
Chapter 2 Provisional chapter Anatomy and Physiology of Hair Anatomy and Physiology of Hair Bilgen Erdoğan ğ AdditionalBilgen Erdo informationan is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/67269 Abstract Hair is one of the characteristic features of mammals and has various functions such as protection against external factors; producing sebum, apocrine sweat and pheromones; impact on social and sexual interactions; thermoregulation and being a resource for stem cells. Hair is a derivative of the epidermis and consists of two distinct parts: the follicle and the hair shaft. The follicle is the essential unit for the generation of hair. The hair shaft consists of a cortex and cuticle cells, and a medulla for some types of hairs. Hair follicle has a continuous growth and rest sequence named hair cycle. The duration of growth and rest cycles is coordinated by many endocrine, vascular and neural stimuli and depends not only on localization of the hair but also on various factors, like age and nutritional habits. Distinctive anatomy and physiology of hair follicle are presented in this chapter. Extensive knowledge on anatomical and physiological aspects of hair can contribute to understand and heal different hair disorders. Keywords: hair, follicle, anatomy, physiology, shaft 1. Introduction The hair follicle is one of the characteristic features of mammals serves as a unique miniorgan (Figure 1). In humans, hair has various functions such as protection against external factors, sebum, apocrine sweat and pheromones production and thermoregulation. The hair also plays important roles for the individual’s social and sexual interaction [1, 2]. -

Hair Histology As a Tool for Forensic Identification of Some Domestic Animal Species
EXCLI Journal 2018;17:663-670 – ISSN 1611-2156 Received: June 28, 2018, accepted: July 02, 2018, published: July 06, 2018 Original article: HAIR HISTOLOGY AS A TOOL FOR FORENSIC IDENTIFICATION OF SOME DOMESTIC ANIMAL SPECIES Yasser A. Ahmed1, Safwat Ali2, Ahmed Ghallab3* 1 Department of Histology, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt 2 Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Minia University, Minia, Egypt 3 Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt * Corresponding author: E-mail: [email protected] http://dx.doi.org/10.17179/excli2018-1478 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). ABSTRACT Animal hair examination at a criminal scene may provide valuable information in forensic investigations. How- ever, local reference databases for animal hair identification are rare. In the present study, we provide differential histological analysis of hair of some domestic animals in Upper Egypt. For this purpose, guard hair of large rumi- nants (buffalo, camel and cow), small ruminants (sheep and goat), equine (horse and donkey) and canine (dog and cat) were collected and comparative analysis was performed by light microscopy. Based on the hair cuticle scale pattern, type and diameter of the medulla, and the pigmentation, characteristic differential features of each animal species were identified. The cuticle scale pattern was imbricate in all tested animals except in donkey, in which coronal scales were identified. The cuticle scale margin type, shape and the distance in between were characteristic for each animal species. -

Vivid Dreams/ Problems Sleeping: Nausea/Upset Stomach: Itching/Rash
Addressing NRT Barriers • Assess the severity of symptoms (Is it tolerable?). • Assess Hx: onset, duration and any troubleshooting that has already taken place. If indicated, get history of these symptoms when not taking these medications. You may also ask how Pt. would normally treat these symptoms. • If symptoms are tolerable àdevelop troubleshooting plan with Pt. Inform Pt. that many symptoms will go away after a few days. Reassess at next visit, but ask Pt. to call if symptoms persist/worsen or become intolerable before next call/visit. • If symptoms are not tolerableàconsider changing products or dosages as applicable. Consult study physician, as needed. Refer Pt. to their personal physician, if needed (e.g., prescription strength creams). • All potential cardiac symptoms should be promptly reported to study physician. Advise Pt. to discontinue NRT when indicated or instructed by study physician. Vivid Dreams/ Problems Sleeping: - Assess if sleep is being disrupted. Is night waking normal for Pt. – what is Pts.’ normal routine? - May try taking patch off at night, keeping in mind cravings may be stronger in the AM. After a couple of nights, try again to wear patch overnight. If using more than 1 patch, may consider only wearing 1 at night. - May try removing patch at night and putting on 2 hours before waking, especially when early morning waking is part of routine. Otherwise, can set an alarm, put on patch, and go back to sleep. - Regulate eating and sleeping patterns and use sleep hygiene tips (relaxation training, avoid caffeine). - Do not smoke or use short-acting NRT within 1-2 hours of bedtime (especially if sleep initiation is the major complaint.) Nausea/upset stomach: - Ask if nausea is only after using gum/lozenge or also after smoking a cigarette. -

Facial Image Comparison Feature List for Morphological Analysis
Disclaimer: As a condition to the use of this document and the information contained herein, the Facial Identification Scientific Working Group (FISWG) requests notification by e-mail before or contemporaneously to the introduction of this document, or any portion thereof, as a marked exhibit offered for or moved into evidence in any judicial, administrative, legislative, or adjudicatory hearing or other proceeding (including discovery proceedings) in the United States or any foreign country. Such notification shall include: 1) the formal name of the proceeding, including docket number or similar identifier; 2) the name and location of the body conducting the hearing or proceeding; and 3) the name, mailing address (if available) and contact information of the party offering or moving the document into evidence. Subsequent to the use of this document in a formal proceeding, it is requested that FISWG be notified as to its use and the outcome of the proceeding. Notifications should be sent to: Redistribution Policy: FISWG grants permission for redistribution and use of all publicly posted documents created by FISWG, provided the following conditions are met: Redistributions of documents, or parts of documents, must retain the FISWG cover page containing the disclaimer. Neither the name of FISWG, nor the names of its contributors, may be used to endorse or promote products derived from its documents. Any reference or quote from a FISWG document must include the version number (or creation date) of the document and mention if the document is in a draft status. Version 2.0 2018.09.11 Facial Image Comparison Feature List for Morphological Analysis 1. -

Nestin Expression in Hair Follicle Sheath Progenitor Cells
Nestin expression in hair follicle sheath progenitor cells Lingna Li*, John Mignone†, Meng Yang*, Maja Matic‡, Sheldon Penman§, Grigori Enikolopov†, and Robert M. Hoffman*¶ *AntiCancer, Inc., 7917 Ostrow Street, San Diego, CA 92111; †Cold Spring Harbor Laboratory, 1 Bungtown Road, Cold Spring Harbor, NY 11724; §Department of Biology, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139-4307; and ‡Stony Brook University, Stony Brook, NY 11794 Contributed by Sheldon Penman, June 25, 2003 The intermediate filament protein, nestin, marks progenitor expression of the neural stem cell protein nestin in hair follicle cells of the CNS. Such CNS stem cells are selectively labeled by stem cells suggests a possible relation. placing GFP under the control of the nestin regulatory se- quences. During early anagen or growth phase of the hair Materials and Methods follicle, nestin-expressing cells, marked by GFP fluorescence in Nestin-GFP Transgenic Mice. Nestin is an intermediate filament nestin-GFP transgenic mice, appear in the permanent upper hair (IF) gene that is a marker for CNS progenitor cells and follicle immediately below the sebaceous glands in the follicle neuroepithelial stem cells (5). Enhanced GFP (EGFP) trans- bulge. This is where stem cells for the hair follicle outer-root genic mice carrying EGFP under the control of the nestin sheath are thought to be located. The relatively small, oval- second-intron enhancer are used for studying and visualizing shaped, nestin-expressing cells in the bulge area surround the the self-renewal and multipotency of CNS stem cells (5–7). hair shaft and are interconnected by short dendrites. The precise Here we report that hair follicle stem cells strongly express locations of the nestin-expressing cells in the hair follicle vary nestin as evidenced by nestin-regulated EGFP expression. -

Smooth Muscle A-Actin Is a Marker for Hair Follicle Dermis in Vivoand in Vitro
Smooth muscle a-actin is a marker for hair follicle dermis in vivo and in vitro COLIN A. B. JAHODA1*, AMANDA J. REYNOLDS2•*, CHRISTINE CHAPONNIER2, JAMES C. FORESTER3 and GIULIO GABBIANI* 1Department of Biological Sciences, University of Dundee, Dundee DD1 4HN, Scotland ^Department of Pathology, University of Geneva, 1211 Geneva 4, Switzerland ^Department of Surgery, Ninewells Hospital and Medical School, Dundee, Scotland * Present address: Department of Biological Sciences, University of Durham, Durham DH1 3LE, England Summary We have examined the expression of smooth muscle cells contained significant quantities of the a-actin a-actin in hair follicles in situ, and in hair follicle isoform. dermal cells in culture by means of immunohisto- The rapid switching on of smooth muscle a-actin chemistry. Smooth muscle a-actin was present in the expression by dermal papilla cells in early culture, dermal sheath component of rat vibrissa, rat pelage contrasts with the behaviour of smooth muscle cells and human follicles. Dermal papilla cells within all in vitro, and has implications for control of ex- types of follicles did not express the antigen. How- pression of the antigen in normal adult systems. The ever, in culture a large percentage of both hair very high percentage of positively marked cultured dermal papilla and dermal sheath cells were stained papilla and sheath cells also provides a novel marker by this antibody. The same cells were negative when of cells from follicle dermis, and reinforces the idea tested with an antibody to desmin. Overall, explant- that they represent a specialized cell population, derived skin fibroblasts had relatively low numbers contributing to the heterogeneity of fibroblast cell of positively marked cells, but those from skin types in the skin dermis, and possibly acting as a regions of high hair-follicle density displayed more source of myofibroblasts during wound healing. -

Neural Control of Facial Sweat Gland Secretion in Horses
Iowa State University Capstones, Theses and Creative Components Dissertations Fall 2019 Neural control of facial sweat gland secretion in horses Lu Liu Follow this and additional works at: https://lib.dr.iastate.edu/creativecomponents Part of the Comparative and Evolutionary Physiology Commons, and the Systems and Integrative Physiology Commons Recommended Citation Liu, Lu, "Neural control of facial sweat gland secretion in horses" (2019). Creative Components. 455. https://lib.dr.iastate.edu/creativecomponents/455 This Creative Component is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Creative Components by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Neural control of facial sweat gland secretion in horses Lu Liu 2019.11.26 BMS 599 Creative Component Biomedical Sciences Department Iowa State University Abstract: For sweat glands, it has been shown that sympathetic neurons express a cholinergic/noradrenergic co-phenotype in their innervation in the trunk of mice.(Schütz et al. 2008). It is unknown if facial sweat glands are innervated the same way. Gustatory sweating is an abnormal sweating condition. People can sweat profusely when eating or drinking, or even thinking about eating or drinking. We hypothesize that the facial sweat glands are controlled differently and can be related to parasympathetic neurons from cranial nerves. Some samples from human donors have been tested, but few sweat glands can be targeted since the donors were older people. Thus, it was decided to use horse tissue for this project to obtain greater numbers of sweat glands on the face. -

GLOSSARY of MEDICAL and ANATOMICAL TERMS
GLOSSARY of MEDICAL and ANATOMICAL TERMS Abbreviations: • A. Arabic • abb. = abbreviation • c. circa = about • F. French • adj. adjective • G. Greek • Ge. German • cf. compare • L. Latin • dim. = diminutive • OF. Old French • ( ) plural form in brackets A-band abb. of anisotropic band G. anisos = unequal + tropos = turning; meaning having not equal properties in every direction; transverse bands in living skeletal muscle which rotate the plane of polarised light, cf. I-band. Abbé, Ernst. 1840-1905. German physicist; mathematical analysis of optics as a basis for constructing better microscopes; devised oil immersion lens; Abbé condenser. absorption L. absorbere = to suck up. acervulus L. = sand, gritty; brain sand (cf. psammoma body). acetylcholine an ester of choline found in many tissue, synapses & neuromuscular junctions, where it is a neural transmitter. acetylcholinesterase enzyme at motor end-plate responsible for rapid destruction of acetylcholine, a neurotransmitter. acidophilic adj. L. acidus = sour + G. philein = to love; affinity for an acidic dye, such as eosin staining cytoplasmic proteins. acinus (-i) L. = a juicy berry, a grape; applied to small, rounded terminal secretory units of compound exocrine glands that have a small lumen (adj. acinar). acrosome G. akron = extremity + soma = body; head of spermatozoon. actin polymer protein filament found in the intracellular cytoskeleton, particularly in the thin (I-) bands of striated muscle. adenohypophysis G. ade = an acorn + hypophyses = an undergrowth; anterior lobe of hypophysis (cf. pituitary). adenoid G. " + -oeides = in form of; in the form of a gland, glandular; the pharyngeal tonsil. adipocyte L. adeps = fat (of an animal) + G. kytos = a container; cells responsible for storage and metabolism of lipids, found in white fat and brown fat. -
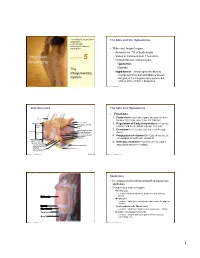
The Integumentary System, but Shares Some of Skin’S Properties
PowerPoint® Lecture Slides The Skin and the Hypodermis prepared by Leslie Hendon University of Alabama, Birmingham • Skin—our largest organ • Accounts for 7% of body weight • Varies in thickness from 1.5–4.4mm C H A P T E R 5 • Divided into two distinct layers • Epidermis The • Dermis Integumentary • Hypodermis—lies deep to the dermis • Composed of areolar and adipose tissues System • Not part of the integumentary system, but shares some of skin’s properties Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Skin Structure The Skin and Hypodermis • Functions 1. Protection—cushions organs and protects from Hair shaft bumps, chemicals, water loss, UV radiation Dermal papillae Epidermis Subpapillary vascular 2. Regulation of body temperature---Capillary Papillary plexus network and sweat glands regulate heat loss layer Pore Appendages of skin 3. Excretion—urea, salts, and water lost through Dermis Reticular Eccrine sweat gland sweat layer Arrector pili muscle Sebaceous (oil) gland Hair follicle 4. Production of vitamin D---Epidermal cells use Hair root UV radiation to synthesize vitamin D Hypodermis (superficial fascia) 5. Sensory reception—Contains sense organs Nervous structures Sensory nerve fiber Dermal vascular plexus associated with nerve endings Lamellar (Pacinian) corpuscle Adipose tissue Hair follicle receptor (root hair plexus) Copyright © 2011 Pearson Education, Inc. Figure 5.1 Copyright © 2011 Pearson Education, Inc. Figure 5.2 Gross structure of skin and underlying tissues. Epidermis • Is composed of keratinized stratified squamous epithelium • Contains four main cell types • Keratinocytes • Location—stratum spinosum; produce keratin a fibrous protein Epidermis • Melanocytes • Location—basal layer; manufacture and secrete the pigment melanin Dermis • Tactile epithelial cells (Merkel cells) Hypodermis • Location—basal layer; attached to sensory nerve endings Deep fascia • Dendritic cells (Langerhans cells) • Location—stratum spinosum; part of immune system; Muscle macrophage-like Copyright © 2011 Pearson Education, Inc. -

Revisiting Hair Follicle Embryology, Anatomy and the Follicular Cycle
etology sm & o C T r f i o c h l o a l n o r Silva et al., J Cosmo Trichol 2019, 5:1 g u y o J Journal of Cosmetology & Trichology DOI: 10.4172/2471-9323.1000141 ISSN: 2471-9323 Review Article Open Access Revisiting Hair Follicle Embryology, Anatomy and the Follicular Cycle Laura Maria Andrade Silva1*, Ricardo Hsieh2, Silvia Vanessa Lourenço3, Bruno de Oliveira Rocha1, Ricardo Romiti4, Neusa Yuriko Sakai Valente4,5, Alessandra Anzai4 and Juliana Dumet Fernandes1 1Department of Dermatology, Magalhães Neto Outpatient Clinic, Professor Edgar Santos School Hospital Complex, The Federal University of Bahia (C-HUPES/UFBa), Salvador/BA-Brazil 2Institute of Tropical Medicine of São Paulo, The University of São Paulo (IMT/USP), São Paulo/SP-Brazil 3Department of Stomatology, School of Dentistry, The University of São Paulo (FO/USP), São Paulo/SP-Brazil 4Department of Dermatology, University of São Paulo Medical School, The University of São Paulo (FMUSP), São Paulo/SP-Brazil 5Department of Dermatology, Hospital of the Public Server of the State of São Paulo (IAMSPE), São Paulo/SP-Brazil *Corresponding author: Laura Maria Andrade Silva, Department of Dermatology, Magalhães Neto Outpatient Clinic, Professor Edgar Santos School Hospital Complex, The Federal University of Bahia (C-HUPES/UFBa) Salvador/BA-Brazil, Tel: +55(71)3283-8380; +55(71)99981-7759; E-mail: [email protected] Received date: January 17, 2019; Accepted date: March 07, 2019; Published date: March 12, 2019 Copyright: © 2019 Silva LMA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. -

1 the Ph of the Human Nail Plate S Murdan*, G
The pH of the human nail plate S Murdan*, G Milcovich and G S Goriparthi Department of Pharmaceutics, UCL School of Pharmacy, 29-39 Brunswick Square, London, WC1N 1AX, UK *corresponding author Tel: +44-2077535810; Fax: +44-2077535942; [email protected] Keywords: nail, pH, surface, tape stripping, washing, gender, acidity The work described in this paper was supported by The School of Pharmacy, University of London (now UCL School of Pharmacy). 1 ABSTRACT In this Chapter, measurements of the nailplate pH are reported. Measurements were conducted in vivo in 37 volunteers with healthy finger and toe nails, using a skin pH meter. The pH of unwashed and washed fingernails and the big toenails was measured and the influence of washing, anatomical site (fingers/toes), side (left/right), finger digit (digits 1-5) and gender were determined. The pH of the nail plate surface was around 5. There was no significant difference between the sides i.e. right or left hand/foot, among the ten fingernails, and between the two great toenails. However, toenails had a significantly higher pH than fingernails. Washing the nails caused an immediate, but transient increase in pH, which was not sustained with time, and pH returned to pre- washing levels within 20 minutes. In males, washing did not significantly influence finger or toe nailplate pH. In females however, washed fingernails had a significantly higher pH than unwashed ones, while there was no difference in the pH of the toenails. The pH of the nail plate interior, measured after tape-stripping, was found to be slightly lower than that at its surface.