Faster Poleward Range Shifts in Moths with More Variable Colour Patterns
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fauna Lepidopterologica Volgo-Uralensis" 150 Years Later: Changes and Additions
©Ges. zur Förderung d. Erforschung von Insektenwanderungen e.V. München, download unter www.zobodat.at Atalanta (August 2000) 31 (1/2):327-367< Würzburg, ISSN 0171-0079 "Fauna lepidopterologica Volgo-Uralensis" 150 years later: changes and additions. Part 5. Noctuidae (Insecto, Lepidoptera) by Vasily V. A n ik in , Sergey A. Sachkov , Va d im V. Z o lo t u h in & A n drey V. Sv ir id o v received 24.II.2000 Summary: 630 species of the Noctuidae are listed for the modern Volgo-Ural fauna. 2 species [Mesapamea hedeni Graeser and Amphidrina amurensis Staudinger ) are noted from Europe for the first time and one more— Nycteola siculana Fuchs —from Russia. 3 species ( Catocala optata Godart , Helicoverpa obsoleta Fabricius , Pseudohadena minuta Pungeler ) are deleted from the list. Supposedly they were either erroneously determinated or incorrect noted from the region under consideration since Eversmann 's work. 289 species are recorded from the re gion in addition to Eversmann 's list. This paper is the fifth in a series of publications1 dealing with the composition of the pres ent-day fauna of noctuid-moths in the Middle Volga and the south-western Cisurals. This re gion comprises the administrative divisions of the Astrakhan, Volgograd, Saratov, Samara, Uljanovsk, Orenburg, Uralsk and Atyraus (= Gurjev) Districts, together with Tataria and Bash kiria. As was accepted in the first part of this series, only material reliably labelled, and cover ing the last 20 years was used for this study. The main collections are those of the authors: V. A n i k i n (Saratov and Volgograd Districts), S. -

Contribution to the Knowledge of the Fauna of Bombyces, Sphinges And
driemaandelijks tijdschrift van de VLAAMSE VERENIGING VOOR ENTOMOLOGIE Afgiftekantoor 2170 Merksem 1 ISSN 0771-5277 Periode: oktober – november – december 2002 Erkenningsnr. P209674 Redactie: Dr. J–P. Borie (Compiègne, France), Dr. L. De Bruyn (Antwerpen), T. C. Garrevoet (Antwerpen), B. Goater (Chandlers Ford, England), Dr. K. Maes (Gent), Dr. K. Martens (Brussel), H. van Oorschot (Amsterdam), D. van der Poorten (Antwerpen), W. O. De Prins (Antwerpen). Redactie-adres: W. O. De Prins, Nieuwe Donk 50, B-2100 Antwerpen (Belgium). e-mail: [email protected]. Jaargang 30, nummer 4 1 december 2002 Contribution to the knowledge of the fauna of Bombyces, Sphinges and Noctuidae of the Southern Ural Mountains, with description of a new Dichagyris (Lepidoptera: Lasiocampidae, Endromidae, Saturniidae, Sphingidae, Notodontidae, Noctuidae, Pantheidae, Lymantriidae, Nolidae, Arctiidae) Kari Nupponen & Michael Fibiger [In co-operation with Vladimir Olschwang, Timo Nupponen, Jari Junnilainen, Matti Ahola and Jari- Pekka Kaitila] Abstract. The list, comprising 624 species in the families Lasiocampidae, Endromidae, Saturniidae, Sphingidae, Notodontidae, Noctuidae, Pantheidae, Lymantriidae, Nolidae and Arctiidae from the Southern Ural Mountains is presented. The material was collected during 1996–2001 in 10 different expeditions. Dichagyris lux Fibiger & K. Nupponen sp. n. is described. 17 species are reported for the first time from Europe: Clostera albosigma (Fitch, 1855), Xylomoia retinax Mikkola, 1998, Ecbolemia misella (Püngeler, 1907), Pseudohadena stenoptera Boursin, 1970, Hadula nupponenorum Hacker & Fibiger, 2002, Saragossa uralica Hacker & Fibiger, 2002, Conisania arida (Lederer, 1855), Polia malchani (Draudt, 1934), Polia vespertilio (Draudt, 1934), Polia altaica (Lederer, 1853), Mythimna opaca (Staudinger, 1899), Chersotis stridula (Hampson, 1903), Xestia wockei (Möschler, 1862), Euxoa dsheiron Brandt, 1938, Agrotis murinoides Poole, 1989, Agrotis sp. -
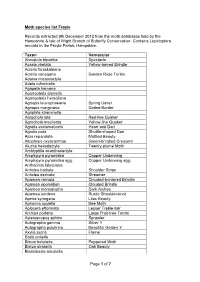
Page 1 of 7 Moth Species List Froyle Records
Moth species list Froyle Records extracted 9th December 2012 from the moth databases held by the Hampshire & Isle of Wight Branch of Butterfly Conservation. Contains Lepidoptera records in the Froyle Parish, Hampshire. Taxon Vernacular Abrostola tripartita Spectacle Acasis viretata Yellow-barred Brindle Acleris forsskaleana Acleris variegana Garden Rose Tortrix Adaina microdactyla Adela rufimitrella Agapeta hamana Agonopterix arenella Agonopterix heracliana Agriopis leucophaearia Spring Usher Agriopis marginaria Dotted Border Agriphila straminella Agrochola lota Red-line Quaker Agrochola macilenta Yellow-line Quaker Agrotis exclamationis Heart and Dart Agrotis puta Shuttle-shaped Dart Alcis repandata Mottled Beauty Allophyes oxyacanthae Green-brindled Crescent Alucita hexadactyla Twenty-plume Moth Amblyptilia acanthadactyla Amphipyra pyramidea Copper Underwing Amphipyra pyramidea agg. Copper Underwing agg. Anthophila fabriciana Anticlea badiata Shoulder Stripe Anticlea derivata Streamer Apamea crenata Clouded-bordered Brindle Apamea epomidion Clouded Brindle Apamea monoglypha Dark Arches Apamea sordens Rustic Shoulder-knot Apeira syringaria Lilac Beauty Aphomia sociella Bee Moth Aplocera efformata Lesser Treble-bar Archips podana Large Fruit-tree Tortrix Asteroscopus sphinx Sprawler Autographa gamma Silver Y Autographa pulchrina Beautiful Golden Y Axylia putris Flame Batia unitella Biston betularia Peppered Moth Biston strataria Oak Beauty Blastobasis adustella Page 1 of 7 Blastobasis lacticolella Cabera exanthemata Common Wave Cabera -

Of India and Kashmir Himalayan Noctuidae
105 | P a g e e-ISSN: 2248-9126 Vol 5|Issue 2| 2015 |105-111. Print ISSN: 2248-9118 Indian Journal of Pharmaceutical Science & Research www.ijpsrjournal.com A TAXONOMIC REPORT OF EIGHT NEWLY RECORDED AND LITTLE KNOWN SPECIES (LEPIDOPTERA) OF INDIA AND KASHMIR HIMALAYAN NOCTUIDAE Mudasir Ahmad Dar*, Jagbir Singh Kirti and ZH Khan2 *1Department of Zoology, Punjabi University, Patiala - 147002, Punjab, India. 2Division of Entomology, SKUAST-K, Shalimar, Jammu and Kashmir, India. ABSTRACT Eight Noctuidae species; Amphipyra herrichschaefferi Hacker and Pek, Catocala amnonfriedbergi Kravchenko et al., Cucullia splendida Cramer, Lacanobia oleracea (Linnaeus), Lophotyna albosignata Moore, Characoma ruficirra Hampson, Simplicia caeneusalis (Walker) and Noctua orbona Hufnagel are newly added to the Himalayan fauna and five to India. In addition, taxonomic notes of all the eight recorded, little known Noctuids are discussed with the description of male and female genitalia. Figures illustrating adults and both sexes’ genitalia are also provided. Keywords: Taxonomic, New records, Himalaya. INTRODUCTION who have worked on the taxonomy of the family Lepidoptera comprising butterflies and moths is Noctuidae are Linnaeus (1758), Fabricius (1793, 1794) and one of the most economically important insect orders. This Hubner (1805) [1-5]. These workers made significant group is suitable for quantitative comparisons between contribution to the taxonomy of family Noctuidae. insect faunas to be valid especially for their abundance, Hampson (1894) was a great worker who contributed species richness, response to vegetation and climate, their tremendously to Indian Noctuidae. His “Fauna of British ease of sampling using light traps, relatively advanced India Moths” included description of 1415 species taxonomy and being common and well known insects due pertaining to 273 genera. -

Results of a Lepidopterological Expedition to North and Northwest Iran in Summer 2007 with New Records for Iran (Lepidoptera) (Plates 19-22)
Esperiana Band 16: 135-165 Schwanfeld, 06. Dezember 2011 ISBN 978-3-938249-01-7 Results of a lepidopterological expedition to North and Northwest Iran in summer 2007 with new records for Iran (Lepidoptera) (plates 19-22) Lutz LEHMANN † & Reza ZAHIRI Abstract: The results of a joint lepidopterological expedition to North and Northwest Iran, povinces of Tehran, Mazandaran, Guilan, Ardabil and Azerbaijan-e-Sharqi, from 20 July to 1 August are presented. More than 508 species of Macrolepidoptera (sensu SEITZ) could be recorded, among them Idaea sericeata (HÜBNER, 1813), Cinglis humifusaria EVERSMANN, 1837, Aplocera uniformata (URBAHN, 1971), Scotopteryx chenopodiata (LINNAEUS, 1758), Triphosa dubitata (LINANEUS, 1758), Abraxas grossulariata (LINNAEUS, 1758), Kemtrognophos ciscaucasica (RJABOV, 1964), Furcula danieli SCHINTLMEISTER, 1998, Eilema lurideola ([ZINCKEN], 1817), Zethes propinquus CHRISTOPH, 1885, Meganola togatulalis (HÜBNER, 1796), Meganola kolbi (DANIEL, 1935), Pseudluperina pozzii (CURÓ, 1883), Mythimna sicula scirpi (DUPONCHEL, 1836) and Noctua interposita (HÜBNER, 1790) new for the fauna of Iran. Additionally, the species of two light traps in the Elburs Mts., province Mazandaran, from the beginning of June, collected by A. PÜTZ, are listed. The genitalia of 16 species, typical habitats, and some living and spread specimens are figured. Zusammenfassung: Die Ergebnisse einer gemeinsamen lepidopterologischen Expedition in den Nord- und Nordwestiran, Provinzen Teheran, Mazandaran, Guilan, Ardabil and Azerbaijan-e-Sharqi, vom -

Scottish Macro-Moth List, 2015
Notes on the Scottish Macro-moth List, 2015 This list aims to include every species of macro-moth reliably recorded in Scotland, with an assessment of its Scottish status, as guidance for observers contributing to the National Moth Recording Scheme (NMRS). It updates and amends the previous lists of 2009, 2011, 2012 & 2014. The requirement for inclusion on this checklist is a minimum of one record that is beyond reasonable doubt. Plausible but unproven species are relegated to an appendix, awaiting confirmation or further records. Unlikely species and known errors are omitted altogether, even if published records exist. Note that inclusion in the Scottish Invertebrate Records Index (SIRI) does not imply credibility. At one time or another, virtually every macro-moth on the British list has been reported from Scotland. Many of these claims are almost certainly misidentifications or other errors, including name confusion. However, because the County Moth Recorder (CMR) has the final say, dubious Scottish records for some unlikely species appear in the NMRS dataset. A modern complication involves the unwitting transportation of moths inside the traps of visiting lepidopterists. Then on the first night of their stay they record a species never seen before or afterwards by the local observers. Various such instances are known or suspected, including three for my own vice-county of Banffshire. Surprising species found in visitors’ traps the first time they are used here should always be regarded with caution. Clerical slips – the wrong scientific name scribbled in a notebook – have long caused confusion. An even greater modern problem involves errors when computerising the data. -

Orman Fakültesi Dergisi
SERİ CİLT SAYI SERIES VOLÜME NUMBER serie A band 56 h e f t 1 2006 SERIE TOME FASCICULE İSTANBUL ÜNİVERSİTESİ ORMAN FAKÜLTESİ DERGİSİ REVIEW OF THE FACULTY OF FORESTRY, UNIVERSITY OF İSTANBUL ZEITSCHRIFT DER FORSTLICHEN FAKULTÂT DER UNIVERSITÂT İSTANBUL REVUE DE LA FACULTE FORESTIERE DE L'UNIVERSITE D'ISTANBUL IIENDEK YÖRESİNİN NOCTUIDAE (LEPİDOPTERA) TÜRLERİ Ar. Gör. Dr. Erol AKKUZU1* Kısa Özet Bu çalışma 2001-2002 yıllarında Sakarya-IIendek’te yapılmış olup, araştırma alanında yaşayan Noctuidae türleri tespit edilmiştir. Arazi çalışmaları öncesinde araştırma alanı ile ilgili (bitki türü, topografya vb.) literatür bilgileri incelenmiştir. Arazide Noctuidae türlerinin yakalanabilmesi için ışık tuzağı ve atrap kullanılmış, örnekler prepare edildikten sonra da teşhis işlemine geçilmiştir. Araştırma sonunda, Noctuidae familyasının 9 alt familyasına ait 21 tür tespit edilmiş, bunlardan orman ağaçlarında zarar yapan türlerin hangileri olduğu ve potansiyel zararları üzerinde durulmuştur. Anahtar Kelimeler: Hendek, Noctuidae, Fauna 1. G İRİŞ Hendek, 58100 ha’lık bir alana sahip olup denizden ortalama yüksekliği 175 m’dir. Araştırma alanımızı kapsayan Hendek Orman İşletme Müdürlüğü 58.873 ha’lık bir yüzölçümüne sahiptir. Bu alanın 29.557 ha’ı (tüm alanın %50,2‘si) ormanlık alan ile kaplıdır. Bu oran Türkiye ortalamasının (%27) ve dünya ortalamasının (%30) oldukça üzerindedir. Çalışma alanında bulunan asli ağaç türlerini yapraklı ağaçlar (Qıtercııs, Fctgııs, Carpinıts, Fraxinııs, Populus, Acer vb. türler) ve iğne yapraklı ağaçlar (Abies bommiilleriana, -

The Lepidoptera of Bucharest and Its Surroundings (Romania)
Travaux du Muséum National d’Histoire Naturelle © 30 Décembre Vol. LIV (2) pp. 461–512 «Grigore Antipa» 2011 DOI: 10.2478/v10191-011-0028-9 THE LEPIDOPTERA OF BUCHAREST AND ITS SURROUNDINGS (ROMANIA) LEVENTE SZÉKELY Abstract. This study presents a synthesis of the current knowledge regarding the Lepidoptera fauna of Bucharest and the surrounding areas within a distance up to 50 kilometers around the Romanian capital. Data about the fauna composition are presented: the results of the research work beginning with the end of the 19th century, as well the results of the research work carried out in the last 15 years. The research initiated and done by the author himself, led to the identification of 180 species which were unknown in the past. Even if the natural habitats from this region have undergone through radical changes in the 20th century, the area still preserves a quite rich and interesting Lepidoptera fauna. The forests provide shelter to rich populations of the hawk moth Dolbina elegans A. Bang-Haas, 1912, one of the rarest Sphingidae in Europe, and some other species with high faunistical and zoogeographical value as: Noctua haywardi (Tams, 1926) (it is new record for the Romanian fauna from this area), Catocala dilecta (Hübner, 1808), Tarachidia candefacta (Hübner, [1831]), Chrysodeixis chalcites (Esper, [1789]), Aedia leucomelas (Linnaeus, 1758), and Hecatera cappa (Hübner, [1809]). We also present and discuss the current status of the protected Lepidoptera species from the surroundings of the Romanian capital for the first time. Résumé. Ce travail représente une synthèse des connaissances actuelles concernant la faune de lépidoptères de Bucarest et de ses zones limitrophes sur un rayon de 50 km autour de la capitale de la Roumanie. -

Kucukosmanoglu: Preliminary Study on Biodiversity of Butterflies in Beykoz Grove of Istanbul, Turkey - 11359
Kucukosmanoglu: Preliminary study on biodiversity of butterflies in Beykoz grove of Istanbul, Turkey - 11359 - PRELIMINARY STUDY ON BIODIVERSITY OF BUTTERFLIES IN BEYKOZ GROVE OF ISTANBUL, TURKEY KUCUKOSMANOGLU, A. Department of Forest Entomology and Protection, Faculty of Forestry, Istanbul University, 34473 Bahcekoy, Sariyer, Istanbul, Turkey (e-mail: [email protected]) (Received 17th Apr 2019; accepted 11th Jul 2019) Abstract. In this study, Lepidoptera species collected by sweep net in Beykoz grove, Istanbul province, Turkey during the years of 2017–2018 was evaluated. A total of 182 specimens were collected in Beykoz. According to identification results, 57 species belonging to 12 families were recorded. The family Noctuidae was represented by the highest number of species (19), followed by Geometridae (16) and Notodontidae (6). Keywords: sweep net, butterflies, biodiversity, fauna, Beykoz Introduction Lepidoptera, butterflies and moths, are very common insects and well known because of their very attractive colours and patterns on their wings. This order is recognized as one of the largest order of insects (Romoser and Stoffolana, 1994). Adults of the majority of extant species feed on nectar, the juice of overripe fruit or other liquids. Larvae of almost all species are phytophagous, and no parts of plants remain unexploited. Because of their phytophagus habits and high reproductive rate, many species are important pests (Gillott, 2005). The ecological-faunistic complex situation of butterflies is important for biodiversity. There are several regional studies to determine the Lepidoptera fauna in Turkey (Akbulut et al., 2003; Akkuzu et al., 2007; Avci, 1997; Beskardes, 2002; Can, 2008; Cebeci, 2003; De Lattin, 1950, 1951; Graves, 1925, 1926; Hakyemez, 1994; Hesselbarth et al., 1995; Kansu, 1963; Kaygin et al., 2009; Kornosor, 1987; Mathew, 1881; Mol, 1977; Okyar and Aktac, 1998, 1999; Rebel, 1903). -

Lepidoptera, Heterocera) Species Diversity in Western Black Sea Region of Turkey
North-Western Journal of Zoology Vol. 5, No. 1, 2009, pp.104-120 P-ISSN: 1584-9074, E-ISSN: 1843-5629 Article No.: 051112 Some aspects of the moth (Lepidoptera, Heterocera) species diversity in Western Black Sea Region of Turkey Zuhal OKYAR1,*, Selcuk YURTSEVER1,*, Nihat AKTAÇ1 and Gökhan ÇAKAN1 1. Biology Department, Faculty of the Science and Arts, 22030 Edirne, Turkey * Corresponding authors: S. Yurtserver, E-mail: [email protected], Z. Oykar, E-mail: [email protected] Abstract. The moth species were investigated in the four distinct types of habitat — coniferous, beech, oak forests and shrubs including 57 different sites — of Western Black Sea Region in Turkey between the years of 2001 and 2004. A total of 207 Lepidoptera species belonging to 164 genera and 11 families was determined. Results showed that the index of diversity in the coniferous habitats (H=0.5592) was significantly higher than those of beech (H=0.3561) and oak forests (H=0.4238), but was not significantly different than those of shrubs (H=0.4921). The pooled species numbers of the coniferous habitats were the highest among the four types of habitat (P<0.001). Moreover, the pooled number of species in the Noctuidae and Geometridae families were significantly higher (P<0.001) than those found in the other Lepidoptera families in the study area. The pooled numbers of the species tended to decrease from June to September during the study months. Key words: Moth, Lepidoptera, Heterocera, diversity, Western Black Sea Region, Turkey. Introduction Some of the later taxonomical studies have been performed in South-Western (Wehrli Due to its distinctive zoogeography and 1932, 1934), Eastern (Wagner 1929, 1930, habitat diversity, Turkey has been one of 1931), and Central Anatolia (Zukowsky the significant survey areas for many 1937, 1938, 1941). -

ARTIGO / ARTÍCULO / ARTICLE a Faunistic Study on the Subfamily Euphorinae (Hymenoptera: Ichneumonoidea, Braconidae) from Iran
ISSN: 1989-6581 Ghahari (2015) www.aegaweb.com/arquivos_entomoloxicos ARQUIVOS ENTOMOLÓXICOS, 14: 149-156 ARTIGO / ARTÍCULO / ARTICLE A faunistic study on the subfamily Euphorinae (Hymenoptera: Ichneumonoidea, Braconidae) from Iran. Hassan Ghahari 1 Department of Plant Protection, Yadegar – e- Imam Khomeini (RAH) Branch, Islamic Azad University, Tehran (IRAN). e-mail: [email protected] Abstract: This paper deals with the species diversity of the subfamily Euphorinae (Hymenoptera: Braconidae) from different regions of Iran. In total 9 species belonging to 6 genera (Dinocampus Foerster, 1863, Leiophron Nees von Esenbeck, 1819, Peristenus Foerster, 1863, Meteorus Haliday, 1835, Microctonus Wesmael, 1835, and Townesilitus Haeselbarth & Loan, 1983) were collected and determined. Key words: Hymenoptera, Braconidae, Euphorinae, fauna, distribution, host, Iran. Resumen: Estudio faunístico de la subfamilia Euphorinae (Hymenoptera: Ichneumonoidea, Braconidae) de Irán. Este trabajo trata de la diversidad de especies de la subfamilia Euphorinae (Hymenoptera: Braconidae) en diferentes regiones de Irán. En total se capturaron e identificaron 9 especies pertenecientes a 6 géneros (Dinocampus Foerster, 1863, Leiophron Nees von Esenbeck, 1819, Peristenus Foerster, 1863, Meteorus Haliday, 1835, Microctonus Wesmael, 1835 y Townesilitus Haeselbarth & Loan, 1983). Palabras clave: Hymenoptera, Braconidae, Euphorinae, fauna, distribución, hospedador, Iran. Recibido: 1 de octubre de 2015 Publicado on-line: 15 de octubre de 2015 Aceptado: 8 de octubre de 2015 Introduction The subfamily Euphorinae (Hymenoptera: Ichneumonoidea: Braconidae) comprises more than 1213 species in 55 genera and 14 tribes of which 456 species are known from the Palaearctic region (Yu et al. 2012). The subfamily possesses a wider host range than any other braconid subfamily, what is reflected in a large morphological diversity (Koldaş et al. -

The Fauna of Insects of Vegetables and Melons of Central Fergana, Especially Its Distribution and Ecology
International Journal of Science and Research (IJSR) ISSN: 2319-7064 ResearchGate Impact Factor (2018): 0.28 | SJIF (2018): 7.426 UDC: 632.7.04 / 08: 635.61 (575.121) The Fauna of Insects of Vegetables and Melons of Central Fergana, Especially Its Distribution and Ecology Zokirov Islomjon Ilkhomjonovich1, Azimov Djalaliddin Azimovich2 1Researcher of Institute of Zoology, Academy of Sciences of Uzbekistan, Candidate of Biological Sciences 2Professor, Academician of the Academy of Sciences of Uzbekistan, Doctor of Biological Sciences Address Uzbekistan, 100125, Tashkent. Yunusabad district, Bogishamol str. 232-b. Tel.: 712890465 e-mail: deputat_zokirov[at]mail.ru Abstract: I.I.Zokirov, D.A.Azimov – for the first time the entomofauna of vegetables and melons of Central Fergana was determined, consisting of 149 species and 6 subspecies belonging to 113 genera, 32 families and 7 orders. 2 species (Trifidaphis phaseoli (Passerini, 1860), Eurydema oleracea (Linnaeus, 1758)) and one subspecies (Aphis frangulae beccabungae Koch, 1855) are new for the entomofauna of Uzbekistan, 19 species for the entomofauna of Central Fergana. 18 invasive species have been found that are found on more than 20 species of cultivated plants, of which 3 species (Phthorimaea operculella (Zeller, 1873), Tuta absoluta (Meyrick, 1917), Myiopardalis pardalina Bigot, 1891) are serious quarantine pests. The main directions of functioning of the phytophagus host system in the following ecological groups were identified: rhizophages — 34 species (21.9%), phyllophages and lymphophages — 120 species (77.4%), anthophages — 32 species (20.6%), carpophages - 15 species (9.7%) and holofagi - 7 species (4.5%). These groups are formed due to trophic competition and existing ecological niches.