Electrochemical Methods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture Content
EMT 518: METHODS IN ENVIROMENTAL ANALYSIS III (2 UNITS) Lecturer: Professor O. Bamgbose SYNOPSIS Electro-analytical method: Potentiometry, Reference electrode – Calomel, Ag/Agcl, indicator electrodes – 1st, 2nd and 3rd order, Metal Electrodes, membrane electrodes – glass electrode, types of liquid junction potential, solid state electrode, potentiometric titration, end point location in potentiometric titration –visual, plot of E/V, plot of derivative curves 1st and 2nd electrogravimetry, fixed potential, constant current, constant cathode potential coulometry: constant current coulometry, coulometric titration. Voltammetry: classical polarography, Description of dropping mercury electrode, condition for polarographic determination, qualitative and quantitative analysis conductance methods: description of limiting ionic conductance, conductance cell, conductomertic titration. Thermal methods: Thermogravimetry, differential thermal analysis (DTA) LECTURE CONTENT POTENTIOMETRY Is a measurement of a given chemical species in an equilibrium system by the use of an electrode, while potentiometric titration is the technique that is used for following the changes in the concentration of chemical species as function of added titrant using an electrode. In both cases a cell is needed and a cell consists of the following: (1) Reference electrode (2) Liquid junction (3) Analyte solution (4) indicator electrode. It is also possible to have a cell without liquid junction. REFERENCE ELECTRODE. In carrying out a potentiometric determination the half cell potential of one electrode must be known which should be constant, reproducible and completely insensitive to the reference electrode and must be fully polarised throughout the duration of the measurement i.e the potential of the reference electrode does not change through the whole measurement. A classical example of reference electrode is the calomel electrode. -

Development of a New Analytical Method for Determination of Veterinary Drug Oxyclozanide by Electrochemical Sensor and Its Application to Pharmaceutical Formulation
chemosensors Article Development of a New Analytical Method for Determination of Veterinary Drug Oxyclozanide by Electrochemical Sensor and Its Application to Pharmaceutical Formulation Ersin Demir 1 and Hulya Silah 2,* 1 Department of Analytical Chemistry, Faculty of Pharmacy, Afyonkarahisar University of Health Sciences, Afyonkarahisar 03200, Turkey; [email protected] 2 Department of Chemistry, Faculty of Art & Science, Bilecik ¸SeyhEdebali University, Bilecik 11210, Turkey * Correspondence: [email protected]; Tel.: +90-228-214-1484 Received: 25 February 2020; Accepted: 26 March 2020; Published: 30 March 2020 Abstract: A novel highly selective, sensitive and simple analytical technique was recommended for the investigation of anthelmintic veterinary drug oxyclozanide based on square wave anodic stripping voltammetry (SWASV) by using a carbon paste electrode (CPE). According to the cyclic voltammetric data, the oxidation and electron transfer processes of oxyclozanide were found as irreversible and adsorption-controlled, respectively. The voltammetric anodic peak response was characterized with respect to pH, accumulation potential, accumulation time, frequency and pulse amplitude, etc. Under these optimized experimental conditions, the anodic peak density of oxyclozanide was linear to oxyclozanide concentrations in the range from 0.058 to 4.00 mg/L. The described electrochemical method was successfully carried out for the oxyclozanide in pharmaceutical formulation and tap water with mean percentage recovery of 101.5 % and 102.2 %, respectively. The results of pharmaceutical formulation studies were statistically compared to the high-performance liquid chromatographic method. Keywords: carbon paste electrode; determination; oxyclozanide; pharmaceutical formulation; voltammetry 1. Introduction Pharmaceuticals are necessary for human and animal health. They are an important class of emerging contaminants in the environment because, after usage, these compounds and their metabolites are widespread in the environment by several mechanisms. -

Physical Electrochemical Software Brochure
Redefining Electrochemical Measurement Physical Electrochemistry Software The Physical Electrochemistry Software is used with a to the limit. You can define the potentials as absolute Gamry Potentiostat to perform in-depth studies of the voltages or by their relationship to the Open-Circuit structure of the electrode interface and the mechanisms Potential. of electrochemical reactions. The software brings Cyclic Voltammetry and other recognized electrochemical The scan rate (mV/s) is determined by the interval between research techniques to the Gamry user. The Physical data points (sample period) and the Step Size (mV): Electrochemistry Software is a useful tool for Step Size() mV fundamental studies, sensor development, small-scale Scan Rate() mV s = energy storage devices, electrophysiology, etc. Sample Period() s The minimum sample period may be as low as 3.3 µs. The This software incorporates the following electrochemical maximum Scan Rate is a function of Step Size. For techniques: example, the maximum Scan Rate with a 2 mV step is 600 V/s. Higher steps provide faster scan rates, but at the ••• Cyclic Voltammetry expense of resolution. Step Sizes greater than 10 mV are ••• Linear Sweep Voltammetry likely to result in unsatisfactory data. ••• Chronoamperometry ••• Repeating Chronoamperometry The Physical Electrochemistry Software can save, and ••• MMuullttiipplleeMultiple-Multiple---StepStep Chronoamperometry display, up to 262,143 data points! The number of CV ••• ChronopotentChronopotentiometryiioommeettrryyiometry cycles that can be displayed is dependent upon the scan ••• Repeating Chronopotentiometry parameters. ••• Chronocoulometry Step Size() mV No. of Cycles =262,143 × ••• Controlled Potential Coulometry Voltage Span of theCV() mV Like most Gamry software, the Physical Electrochemistry Software and a Gamry Potentiostat use the Framework for data acquisition and the Echem Analyst for data analysis. -

Stationary Electrode Voltammetry and Chronoamperometry in an Alkali Metal Carbonate-Borate Melt
AN ABSTRACT OF THE THESIS OF DARRELL GEORGE PETCOFF for the Doctor of Philosophy (Name of student) (Degree) in Analytical Chemistry presented onC (O,/97 (Major) (Date) Title: STATIONARY ELECTRODE VOLTAMMETRY AND CHRONOAMPEROMETRY IN AN ALKALI METAL CARBONATE - BORATE. MFT T Abstract approved: Redacted for Privacy- Drir. reund The electrochemistry of the lithium-potassium-sodium carbonate-borate melt was explored by voltammetry and chrono- amperometry. In support of this, a controlled-potential polarograph and associated hardware was constructed.Several different types of reference electrodes were tried before choosing a porcelain mem- brane electrode containing a silver wire immersed in a silver sulfate melt.The special porcelain compounded was used also to construct a planar gold disk electrode.The theory of stationary electrode polarography was summarized and denormalized to provide an over- all view. A new approach to the theory of the cyclic background current was also advanced. A computer program was written to facilitate data processing.In addition to providing peak potentials, currents, and n-values, the program also resolves overlapping peaks and furnishes plots of both processed and unprocessed data. Rapid-scan voltammetry was employed to explore the electro- chemical behavior of Zn, Co, Fe, Tl, Sb, As, Ni, Sn, Cd, Te, Bi, Cr, Pb, Cu, and U in the carbonate-borate melt. Most substances gave reasonably well-defined peaks with characteristic peak potentials and n-values.Metal deposition was commonly accompanied by adsorp- tion prepeaks indicative of strong adsorption, and there was also evi- dence of a preceding chemical reaction for several elements, sug- gesting decomplexation before reduction. -

Analytical Applications of Nuclear Techniques
ANALYTICAL APPLICATIONS OF NUCLEAR TECHNIQUES ANALYTICAL APPLICATIONS OF NUCLEAR TECHNIQUES The following States are Members of the International Atomic Energy Agency: AFGHANISTAN GUATEMALA PERU ALBANIA HAITI PHILIPPINES ALGERIA HOLY SEE POLAND ANGOLA HONDURAS PORTUGAL ARGENTINA HUNGARY QATAR ARMENIA ICELAND REPUBLIC OF MOLDOVA AUSTRALIA INDIA ROMANIA AUSTRIA INDONESIA AZERBAIJAN IRAN, ISLAMIC REPUBLIC OF RUSSIAN FEDERATION BANGLADESH IRAQ SAUDI ARABIA BELARUS IRELAND SENEGAL BELGIUM ISRAEL SERBIA AND MONTENEGRO BENIN ITALY SEYCHELLES BOLIVIA JAMAICA SIERRA LEONE BOSNIA AND HERZEGOVINA JAPAN SINGAPORE BOTSWANA JORDAN SLOVAKIA BRAZIL KAZAKHSTAN SLOVENIA BULGARIA KENYA SOUTH AFRICA BURKINA FASO KOREA, REPUBLIC OF SPAIN CAMEROON KUWAIT CANADA KYRGYZSTAN SRI LANKA CENTRAL AFRICAN LATVIA SUDAN REPUBLIC LEBANON SWEDEN CHILE LIBERIA SWITZERLAND CHINA LIBYAN ARAB JAMAHIRIYA SYRIAN ARAB REPUBLIC COLOMBIA LIECHTENSTEIN TAJIKISTAN COSTA RICA LITHUANIA THAILAND CÔTE D’IVOIRE LUXEMBOURG THE FORMER YUGOSLAV CROATIA MADAGASCAR REPUBLIC OF MACEDONIA CUBA MALAYSIA TUNISIA CYPRUS MALI TURKEY CZECH REPUBLIC MALTA DEMOCRATIC REPUBLIC MARSHALL ISLANDS UGANDA OF THE CONGO MAURITIUS UKRAINE DENMARK MEXICO UNITED ARAB EMIRATES DOMINICAN REPUBLIC MONACO UNITED KINGDOM OF ECUADOR MONGOLIA GREAT BRITAIN AND EGYPT MOROCCO NORTHERN IRELAND EL SALVADOR MYANMAR UNITED REPUBLIC ERITREA NAMIBIA OF TANZANIA ESTONIA NETHERLANDS UNITED STATES OF AMERICA ETHIOPIA NEW ZEALAND URUGUAY FINLAND NICARAGUA UZBEKISTAN FRANCE NIGER GABON NIGERIA VENEZUELA GEORGIA NORWAY VIETNAM GERMANY PAKISTAN YEMEN GHANA PANAMA ZAMBIA GREECE PARAGUAY ZIMBABWE The Agency’s Statute was approved on 23 October 1956 by the Conference on the Statute of the IAEA held at United Nations Headquarters, New York; it entered into force on 29 July 1957. The Headquarters of the Agency are situated in Vienna. Its principal objective is “to accelerate and enlarge the contribution of atomic energy to peace, health and prosperity throughout the world’’. -

Reference Materials for Microanalytical Nuclear Techniques
IAEA-TECDOC-1295 Reference materials for microanalytical nuclear techniques Final report of a co-ordinated research project 1994–1999 June 2002 The originators of this publication in the IAEA were: Industrial Applications and Chemistry Section and IAEA Laboratories, Seibersdorf International Atomic Energy Agency Wagramer Strasse 5 P.O. Box 100 A-1400 Vienna, Austria REFERENCE MATERIALS FOR MICROANALYTICAL NUCLEAR TECHNIQUES IAEA, VIENNA, 2002 IAEA-TECDOC-1295 ISSN 1011–4289 © IAEA, 2002 Printed by the IAEA in Austria June 2002 FOREWORD In 1994 the IAEA established a Co-ordinated Research Project (CRP) on Reference Materials for Microanalytical Nuclear Techniques as part of its efforts to promote and strengthen the use of nuclear analytical technologies in Member States with the specific aim of improving the quality of analysis in nuclear, environmental and biological materials. The objectives of this initiative were to: identify suitable biological reference materials which could serve the needs for quality control in micro-analytical techniques, evaluate existing CRMs for use in micro-analytical investigations, evaluate appropriate sample pre-treatment procedures for materials being used for analysis with micro-analytical techniques, identify analytical techniques which can be used for characterisation of homogeneity determination, and apply such techniques to the characterization of candidate reference materials for use with micro-analytical techniques. The CRP lasted for four years and seven laboratories and the IAEA’s Laboratories in Seibersdorf participated. A number of materials including the candidate reference materials IAEA 338 (lichen) and IAEA 413 (single cell algae, elevated level) were evaluated for the distribution of elements such as Cl, K, Ca, Cr, Mn, Fe, Zn, As, Br, Rb, Cd, Hg and Pb. -

COULOMETRY for the DETERMINATION of URANIUM and PLUTONIUM: PAST and PRESENT by M.K
BARC/2012/E/001 BARC/2012/E/001 COULOMETRY FOR THE DETERMINATION OF URANIUM AND PLUTONIUM: PAST AND PRESENT by M.K. Sharma, J.V. Kamat, A.S. Ambolikar, J.S. Pillai and S.K. Aggarwal Fuel Chemistry Division 2012 BARC/2012/E/001 GOVERNMENT OF INDIA ATOMIC ENERGY COMMISSION BARC/2012/E/001 COULOMETRY FOR THE DETERMINATION OF URANIUM AND PLUTONIUM: PAST AND PRESENT by M.K. Sharma, J.V. Kamat, A.S. Ambolikar, J.S. Pillai and S.K. Aggarwal Fuel Chemistry Division BHABHA ATOMIC RESEARCH CENTRE MUMBAI, INDIA 2012 BARC/2012/E/001 BIBLIOGRAPHIC DESCRIPTION SHEET FOR TECHNICAL REPORT (as per IS : 9400 - 1980) 01 Security classification : Unclassified 02 Distribution : External 03 Report status : New 04 Series : BARC External 05 Report type : Technical Report 06 Report No. : BARC/2012/E/001 07 Part No. or Volume No. : 08 Contract No. : 10 Title and subtitle : Coulometry for the determination of uranium and plutonium: past and present 11 Collation : 34 p., 2 figs., 7 tabs. 13 Project No. : 20 Personal author(s) : M.K. Sharma; J.V. Kamat; A.S. Ambolikar; J.S. Pillai; S.K. Aggarwal 21 Affiliation of author(s) : Fuel Chemistry Division, Bhabha Atomic Research Centre, Mumbai 22 Corporate author(s) : Bhabha Atomic Research Centre, Mumbai - 400 085 23 Originating unit : Fuel Chemistry Division, BARC, Mumbai 24 Sponsor(s) Name : Department of Atomic Energy Type : Government Contd... BARC/2012/E/001 30 Date of submission : December 2011 31 Publication/Issue date : January 2012 40 Publisher/Distributor : Head, Scientific Information Resource Division, Bhabha Atomic Research Centre, Mumbai 42 Form of distribution : Hard copy 50 Language of text : English 51 Language of summary : English, Hindi 52 No. -

Plutonium Analysis from Controlled-Potential Coulometry for the Certification of the MP3 Standard Material
P5_14 Plutonium analysis from controlled-potential coulometry for the certification of the MP3 standard material. A. Ruas, V. Dalier, J. Pivato CEA-Marcoule BP 17171 30207 Bagnols-sur-Cèze Cedex, France [email protected] Abstract – For contributing to the certification of the new metal plutonium reference material (MP3), controlled-potential coulometry (CPC) has many advantages: it is a high accuracy absolute chemical analysis technique. Many studies are now conducted on plutonium solutions, to improve the operating conditions and the current apparatus, for mass determination with a precision of 0.1%. The different experimental preliminary results are discussed and the apparatus described. The coulometry cell assembly comprises a motor connected to a stirrer designed to prevent splashing, an inlet tube for inert gas, three electrodes, and a thermocouple for measuring the temperature. The measuring system includes a potentiostat, a CPU, a calibrated current generator, a temperature indicator and a voltmeter, all maintained at a constant temperature. Current integration is made by electronic components, introduced in the potentiostat and the CPU. + − + INTRODUCTION Pu 4 + e ↔ Pu 3 (1) Experimental electrolysis is performed using a Coulometry is an assay method in which the metal electrode with a large surface area quantity of the element analyzed is determined (working electrode) immersed in the test by measuring a quantity of electricity; under solution. The quantity of electricity used for the certain conditions it is capable of providing a conversion is related to the quantity matter in very accurate determination of the plutonium solution by Faraday’s law of electrolysis: mass concentration. The advantage of this M method is that it is absolute and uses only small m = ⋅Q (2) masses of material. -

School of Engineering and Science to My Beloved Parents Abstract
Hybrid Organic-Inorganic Polyoxometalates Functionalized by Diorganotin Groups by Firasat Hussain A thesis submitted in partial ful¯llment of the requirements for the degree of Doctor of Philosophy Approved, Thesis Committee: Prof. Ulrich Kortz (Mentor), IUB Prof. Ryan M Richards, IUB Dr. Michael H Dickman, IUB Prof. Michael T Pope Georgetown University, U.S.A. Prof. Emmanuel Cadot Universit¶ede Versailles, France Date of defense: 19 May 2006 School of Engineering and Science To my beloved parents Abstract Polyoxometalates (POMs) are a well-known class of inorganic metal-oxygen clusters with an unmatched structural variety combined with a multitude of properties. The search for novel POMs is predominantly driven by exciting catalytic, medicinal, material science and bioscience applications. However, the mechanism of action of most polyoxoanions is not selective towards a speci¯c target. In order to improve selectivity it appears highly desirable to attach organic functionalities covalently to the surface of polyoxoanions. The hydrolytic stability of the Sn-C bond enables the synthesis of a novel class of polyoxoanions via attachment of organometallic functionalities based on Sn(IV) to the surface of lacunary polyoxoanion precursors. III III By reacting (CH3)2SnCl2 with Na9(®-XW9O33) (X = As , Sb ) in aqueous acidic medium leads to the formation of 2-D solid-state structures with inorganic and organic surface, which are rare examples of discrete polyoxoanions. (CsNa4f(Sn(CH3)2)3O(H2O)4 (¯-AsW9O33)g¢5H2O)1 (CsNa-1) and the isostructural (CsNa4[(Sn(CH3)2)3O(H2O)4( ¯-SbW9O33)]¢5H2O)1 (CsNa-2)It has been synthesized and characterized by multinu- clear NMR spectroscopy, FTIR spectroscopy and elemental analysis. -
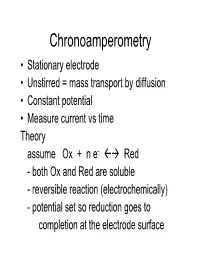
Chronoamperometry
Chronoamperometry • Stationary electrode • Unstirred = mass transport by diffusion • Constant potential • Measure current vs time Theory assume Ox + n e- Red - both Ox and Red are soluble - reversible reaction (electrochemically) - potential set so reduction goes to completion at the electrode surface Components of output signal in Chronoamperometry IFar decreases because Ox used up at electrode surface and Ox is only replenished by diffusion Faradaic current (IFar) follows Cottrell equation I Capacitive current (Icap) (current) decays exponentially for a constant applied potential t (time) Icap is high as electrode capacitive layer charges up, then drops off Processes perturbing system can cause data to differ from Cottrell Equation 1) Capacitive Current – charging current is exponential as shown -kt Icap = e Note: Capacitive current decreases more rapidly than Faradaic current so at longer times the ratio IFar/Icap is larger 2) Occurrence of coupled chemical reactions e.g. Ox + n e- Red 2 Red A A + n e- B Affects the shape of the current-time curve Chronoamperometry Applications • Can measure concentration by measuring I vs conc. at any fixed time • Can analyze the shape of the current-time curve in order to study coupled chemical reactions • There are better ways to do both of these with more modern techniques • Chronoamperometry is important because it is a fundamental method on which other techniques are based Chronopotentiometry • Stationary electrode • Unstirred = mass transport by diffusion • Constant current applied between -

Development and Evaluation of a Calibration Free Exhaustive Coulometric Detection System for Remote Sensing
University of Louisville ThinkIR: The University of Louisville's Institutional Repository Electronic Theses and Dissertations 5-2014 Development and evaluation of a calibration free exhaustive coulometric detection system for remote sensing. Thomas James Roussel University of Louisville Follow this and additional works at: https://ir.library.louisville.edu/etd Part of the Mechanical Engineering Commons Recommended Citation Roussel, Thomas James, "Development and evaluation of a calibration free exhaustive coulometric detection system for remote sensing." (2014). Electronic Theses and Dissertations. Paper 1238. https://doi.org/10.18297/etd/1238 This Doctoral Dissertation is brought to you for free and open access by ThinkIR: The University of Louisville's Institutional Repository. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of ThinkIR: The University of Louisville's Institutional Repository. This title appears here courtesy of the author, who has retained all other copyrights. For more information, please contact [email protected]. DEVELOPMENT AND EVALUATION OF A CALIBRATION FREE EXHAUSTIVE COULOMETRIC DETECTION SYSTEM FOR REMOTE SENSING by Thomas James Roussel, Jr. B.A., University of New Orleans, 1993 B.S., Louisiana Tech University, 1997 M.S., Louisiana Tech University, 2001 A Dissertation Submitted to the Faculty of the J. B. Speed School of Engineering of the University of Louisville in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Department of Mechanical Engineering University of Louisville Louisville, Kentucky May 2014 Copyright 2014 by Thomas James Roussel, Jr. All rights reserved DEVELOPMENT AND EVALUATION OF A CALIBRATION FREE EXHAUSTIVE COULOMETRIC DETECTION SYSTEM FOR REMOTE SENSING By Thomas James Roussel, Jr. -

High Speed Controlled Potential Coulometry
c1CYCLIC CHELONO, DIFPU- c2SOLVE GENERATED EQUA- 903 FORMAT (5HRR =, F10.5, SION CONTROLL, PLANE TION BEGIN AT 96 READ 8HFRACT =, F10.5) ELECTRODE, READ IN K IN NOSIG FOR ACCURACY GO TO 920 NOSIG RR FRACT, TWO 96 IF(M- 1)300,100,102 300 PRINT905 SOLUBLE ElPECIES 100 Z=Y 905 FORRSAT (2X,5HEItROR) READ 900,K,NOSlG, RR, M=M+l 920 STOP FRACT 102 IF (Z) 98,200,99 EXD DIMENSION X (100),T (1 00) , 98 IF (Y) 71,200,73 END R(100) 99 IF (Y) 73,200,71 C GENERATION OF EQUA- 71 T(N) = T(N) + 10.0 **(-LA) LITERATURE CITED TIONS GO TO 10 (1) Alden, J. R., Chambers, J. Q., Adams, DO200N = 1,K 73 T(N) = T(K) - 10.0 **(-LA) R. N., J. Electroanal. Chem. 5, 152 T(N) = 0.0 LA=LA+I (1963). M=l 199 IF (NOSIG - LA) 300,200,71 (2) Bard, A. J., ANAL. CHEM. 33, 11 (1961). LA = 0 200 CONTIXUE (3) Churchill, R. V., “Operational Mathe- 10 DO 80 I = 1,N c3EQUATION SOLVED PRINT matics,” p. 39, McGraw-Hill, New York, SUM = 0.0 ANSWER 1958. DO 60 J = I,N DO201 J = 1,K,2 (4) Galus, Z., Lee, H. Y., Adams, R. N., = 201 R(J) = T(J)/T(J 1) J. Electroanal. Chem. 5, 17 (1963). 60 SUM SUM -- T(J) + (5) Murra,y, R. W., Reilley, C. N., Ibid., X(1) = SQRTF(SUM) PRINT 903, RR, FRACT 3, 182 (1962). 80 CONTIXUE PRINT 901 (6) Piette, L.