Cycloalkanes Heats of Combustion for Cycloalkanes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Conformations of Cycloalkanes
The Conformations of Cycloalkanes Ring-containing structures are a common occurrence in organic chemistry. We must, therefore spend some time studying the special characteristics of the parent cycloalkanes. Cyclical connectivity imposes constraints on the range of motion that the atoms in rings can undergo. Cyclic molecules are thus more rigid than linear or branched alkanes because cyclic structures have fewer internal degrees of freedom (that is, the motion of one atom greatly influences the motion of the others when they are connected in a ring). In this lesson we will examine structures of the common ring structures found in organic chemistry. The first four cycloalkanes are shown below. cyclopropane cyclobutane cyclopentane cyclohexane The amount of energy stored in a strained ring is estimated by comparing the experimental heat of formation to the calculated heat of formation. The calculated heat of formation is based on the notion that, in the absence of strain, each –CH2– group contributes equally to the heat of formation, in line with the behavior found for the acyclic alkanes (i.e., open chains). Thus, the calculated heat of formation varies linearly with the number of carbon atoms in the ring. Except for the 6-membered ring, the experimental values are found to have a more positive heat of formation than the calculated value owning to ring strain. The plots of calculated and experimental enthalpies of formation and their difference (i.e., ring strain) are seen in the Figure. The 3-membered ring has about 27 kcal/mol of strain. It can be seen that the 6-membered ring possesses almost no ring strain. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Cyclobutane Derivatives in Drug Discovery
Cyclobutane Derivatives in Drug Discovery Overview Key Points Unlike larger and conformationally flexible cycloalkanes, Cyclobutane adopts a rigid cyclobutane and cyclopropane have rigid conformations. Due to the ring strain, cyclobutane adopts a rigid puckered puckered conformation Offer ing advantages on (~30°) conformation. This unique architecture bestowed potency, selectivity and certain cyclobutane-containing drugs with unique pharmacokinetic (PK) properties. When applied appropriately, cyclobutyl profile. scaffolds may offer advantages on potency, selectivity and pharmacokinetic (PK) profile. Bridging Molecules for Innovative Medicines 1 PharmaBlock designs and Cyclobutane-containing Drugs synthesizes over 1846 At least four cyclobutane-containing drugs are currently on the market. cyclobutanes, and 497 Chemotherapy carboplatin (Paraplatin, 1) for treating ovarian cancer was cyclobutane products are prepared to lower the strong nephrotoxicity associated with cisplatin. By in stock. CLICK HERE to replacing cisplatin’s two chlorine atoms with cyclobutane-1,1-dicarboxylic find detailed product acid, carboplatin (1) has a much lower nephrotoxicity than cisplatin. On information on webpage. the other hand, Schering-Plough/Merck’s hepatitis C virus (HCV) NS3/4A protease inhibitor boceprevir (Victrelis, 2) also contains a cyclobutane group in its P1 region. It is 3- and 19-fold more potent than the 1 corresponding cyclopropyl and cyclopentyl analogues, respectively. Androgen receptor (AR) antagonist apalutamide (Erleada, 4) for treating castration-resistant prostate cancer (CRPC) has a spirocyclic cyclobutane scaffold. It is in the same series as enzalutamide (Xtandi, 3) discovered by Jung’s group at UCLA in the 2000s. The cyclobutyl- (4) and cyclopentyl- derivative have activities comparable to the dimethyl analogue although the corresponding six-, seven-, and eight-membered rings are slightly less 2 active. -

Comparing Models for Measuring Ring Strain of Common Cycloalkanes
The Corinthian Volume 6 Article 4 2004 Comparing Models for Measuring Ring Strain of Common Cycloalkanes Brad A. Hobbs Georgia College Follow this and additional works at: https://kb.gcsu.edu/thecorinthian Part of the Chemistry Commons Recommended Citation Hobbs, Brad A. (2004) "Comparing Models for Measuring Ring Strain of Common Cycloalkanes," The Corinthian: Vol. 6 , Article 4. Available at: https://kb.gcsu.edu/thecorinthian/vol6/iss1/4 This Article is brought to you for free and open access by the Undergraduate Research at Knowledge Box. It has been accepted for inclusion in The Corinthian by an authorized editor of Knowledge Box. Campring Models for Measuring Ring Strain of Common Cycloalkanes Comparing Models for Measuring R..ing Strain of Common Cycloalkanes Brad A. Hobbs Dr. Kenneth C. McGill Chemistry Major Faculty Sponsor Introduction The number of carbon atoms bonded in the ring of a cycloalkane has a large effect on its energy. A molecule's energy has a vast impact on its stability. Determining the most stable form of a molecule is a usefol technique in the world of chemistry. One of the major factors that influ ence the energy (stability) of cycloalkanes is the molecule's ring strain. Ring strain is normally viewed as being directly proportional to the insta bility of a molecule. It is defined as a type of potential energy within the cyclic molecule, and is determined by the level of "strain" between the bonds of cycloalkanes. For example, propane has tl1e highest ring strain of all cycloalkanes. Each of propane's carbon atoms is sp3-hybridized. -

Text Related to Segment 5.02 ©2002 Claude E. Wintner from the Previous Segment We Have the Value of the Heat of Combustion Of
Text Related to Segment 5.02 ©2002 Claude E. Wintner From the previous segment we have the value of the heat of combustion of an "unstrained" methylene unit as -157.4 kcal/mole. On this basis one would predict that combustion of "unstrained" cyclopropane should liberate 3 X 157.4 = 472.2 kcal/mole; however, when cyclopropane is burned, a value of 499.8 kcal/mole is measured for the actual heat of combustion. The difference of 27.6 kcal/mole is interpreted as representing the higher internal energy ("strain energy") of real cyclopropane as compared to a postulated strain-free "model." ("Model" in this usage speaks of a proposal, or postulate.) These facts are graphed conveniently on an energy diagram as follows: "Strain Energy" represents the error units: kcal/mole not to scale in the "strain-free model" real cyclopropane + 4.5 O2 27.6 "strain-free model" of cyclopropane 499.8 observed heat of combustion + 4.5 O2 472.2 3CO2 + 3H2O heat of combustion predicted for "strain-free model" Note that this estimate of the strain energy in cyclopropane amounts to 9.2 kcal/mole of strain per methylene group (dividing 27.6 by 3, because of the three carbon atoms). Comparable values in kcal/mole for other small and medium rings are given in the following table. As one might expect, in general the strain decreases as the ring is enlarged. units: kcal/mole n Total Strain Strain per CH2 3 27.6 9.2 (CH2)n 4 26.3 6.6 5 6.2 1.2 6 0.1 0.0 ! 7 6.2 0.9 8 9.7 1.2 9 12.6 1.4 10 12.4 1.2 12 4.1 0.3 15 1.9 0.1 Without entering into a discussion of the relevant bonding concepts here, and instead relying on geometry alone, interpretation of the source of the strain energy in cyclopropane and cyclobutane is to some extent self-evident. -

Recent Advances in the Total Synthesis of Cyclobutane-Containing Natural Products Cite This: Org
Volume 7 | Number 1 | 7 January 2020 ORGANIC CHEMISTRY FRONTIERS rsc.li/frontiers-organic ORGANIC CHEMISTRY FRONTIERS View Article Online REVIEW View Journal | View Issue Recent advances in the total synthesis of cyclobutane-containing natural products Cite this: Org. Chem. Front., 2020, 7, 136 Jinshan Li,†a Kai Gao, †a Ming Bianb and Hanfeng Ding *a,c Complex natural products bearing strained cyclobutane subunits, including terpenoids, alkaloids and steroids, not only display fascinating architectures, but also show potent biological activities. Due to their unique structures as critical core skeletons in these molecules, a variety of new strategies for the con- Received 24th September 2019, struction of cyclobutane rings have greatly emerged during the last decade. In this review, we wish to Accepted 11th November 2019 summarize the recent progress in the cyclobutane-containing natural product synthesis with an emphasis DOI: 10.1039/c9qo01178a on disconnection tactics employed to forge the four-membered rings, aiming to provide a complement rsc.li/frontiers-organic to existing reviews. 1. Introduction stereoselectively, poses significant challenges in synthetic chemistry. On the other hand, cyclobutanes readily undergo a In the class of strained carbocycles, cyclobutanes have been number of ring-opening reactions by virtue of their tendency known as intriguing structural motifs for more than one to release inherent strain energies. In some cases, however, century but remained relatively less explored in parallel with striking ring strains can be dramatically reduced by the instal- their homologues.1 Due to the highly strained ring systems (ca. lation of a gem-dialkyl substituent (through the Thorpe–Ingold − 26.7 kcal mol 1), construction of cyclobutane rings, especially effect),2 a carbonyl group, a heteroatom, or other functional- ities (Fig. -

Chapter 2: Alkanes Alkanes from Carbon and Hydrogen
Chapter 2: Alkanes Alkanes from Carbon and Hydrogen •Alkanes are carbon compounds that contain only single bonds. •The simplest alkanes are hydrocarbons – compounds that contain only carbon and hydrogen. •Hydrocarbons are used mainly as fuels, solvents and lubricants: H H H H H H H H H H H H C H C C H C C C C H H C C C C C H H H C C H H H H H H CH2 H CH3 H H H H CH3 # of carbons boiling point range Use 1-4 <20 °C fuel (gasses such as methane, propane, butane) 5-6 30-60 solvents (petroleum ether) 6-7 60-90 solvents (ligroin) 6-12 85-200 fuel (gasoline) 12-15 200-300 fuel (kerosene) 15-18 300-400 fuel (heating oil) 16-24 >400 lubricating oil, asphalt Hydrocarbons Formula Prefix Suffix Name Structure H CH4 meth- -ane methane H C H H C H eth- -ane ethane 2 6 H3C CH3 C3H8 prop- -ane propane C4H10 but- -ane butane C5H12 pent- -ane pentane C6H14 hex- -ane hexane C7H16 hept- -ane heptane C8H18 oct- -ane octane C9H20 non- -ane nonane C10H22 dec- -ane decane Hydrocarbons Formula Prefix Suffix Name Structure H CH4 meth- -ane methane H C H H H H C2H6 eth- -ane ethane H C C H H H H C H prop- -ane propane 3 8 H3C C CH3 or H H H C H 4 10 but- -ane butane H3C C C CH3 or H H H C H 4 10 but- -ane butane? H3C C CH3 or CH3 HydHrydorcocaarrbobnos ns Formula Prefix Suffix Name Structure H CH4 meth- -ane methane H C H H H H C2H6 eth- -ane ethane H C C H H H H C3H8 prop- -ane propane H3C C CH3 or H H H C H 4 10 but- -ane butane H3C C C CH3 or H H H C H 4 10 but- -ane iso-butane H3C C CH3 or CH3 HydHrydoroccarbrobnsons Formula Prefix Suffix Name Structure H H -

SAFETY DATA SHEET Cyclopentane
SAFETY DATA SHEET Cyclopentane Section 1. Identification GHS product identifier : Cyclopentane Chemical name : cyclopentane Other means of : cyclopentane (dot); pentamethylene identification Product use : Synthetic/Analytical chemistry. Synonym : cyclopentane (dot); pentamethylene SDS # : 001124 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE LIQUIDS - Category 2 substance or mixture AQUATIC HAZARD (ACUTE) - Category 3 AQUATIC HAZARD (LONG-TERM) - Category 3 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : Highly flammable liquid and vapor. May form explosive mixtures in Air. Harmful to aquatic life with long lasting effects. Precautionary statements General : Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Prevention : Wear protective gloves. Wear eye or face protection. Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. Use explosion- proof electrical, ventilating, lighting and all material-handling equipment. Use only non- sparking tools. Take precautionary measures against static discharge. Keep container tightly closed. Avoid release to the environment. Response : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water or shower. Storage : Store in a well-ventilated place. Keep cool. Disposal : Dispose of contents and container in accordance with all local, regional, national and international regulations. Hazards not otherwise : None known. -
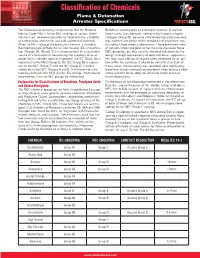
Classification of Chemicals
Classification of Chemicals Flame & Detonation Arrester Specifications PROTECTOSEAL ® The Protectoseal Company recommends that the National Butadiene would qualify as a Group D material. In each of Electric Code (NEC) Article 500, rankings of various chemi - these cases, the chemicals were primarly listed in a higher cals be used, whenever possible, to determine the suitability category (Group B), because of relatively high pressure read - of a detonation arrester for use with a particular chemical. ings noted in one phase of the standard test procedure con - When no NEC rating of the particular chemical is available, ducted by Underwriters Laboratories. These pressures were the International Electrotechnical Commission (IEC) classifica - of concern when categorizing the chemicals because these tion (Groups IIA, IIB and IIC) is recommended as a secondary NEC groupings are also used as standard indicators for the source of information for determining the suitability of an ar - design strength requirements of electrical boxes, apparatus, rester for its intended service. In general, the IEC Group IIA is etc. that must withstand the pressures generated by an igni - equivalent to the NEC Group D; the IEC Group IIB is equiva - tion within the container. It should be noted that, in each of lent to the NEC Group C; and the IEC Group IIC includes these cases, the test pressures recorded were significantly chemicals in the NEC Groups A and B. In the event of a dis - lower than those commonly encountered when testing a deto - crepancy between the NEC and the IEC ratings, Protectoseal nation arrester for its ability to withstand stable and over - recommends that the NEC groups be referenced. -

Today's Class Will Focus on the Conformations and Energies of Cycloalkanes
Name ______________________________________ Today's class will focus on the conformations and energies of cycloalkanes. Section 6.4 of the text provides an introduction. 1 Make a model of cyclopentane. Use the 2-piece "lockable" bond connectors — these rotate more easily than the smaller ones. You may need to twist them to "lock" so that they don't pop apart. The text shows two conformations of this ring — the envelope and the half-chair. Why is cyclopentane non-planar? The two conformations are below. In the envelope, 4 Cs are in a plane; in the half-chair 3 Cs are in a plane. Th first conformer is easy. To get your model to look like the second one, start with a planar ring, keep the "front" 3 Cs in the plane, and twist the "back" C–C bond so that one C goes above that plane and the other goes below. Add Hs to each of these structures — no wedges and dashes here — these are "perspective" drawings (like the drawings of chair cyclohexane rings on p 197). Just use your model and sketch the Hs where they appear to be. fast If we were to replace one H in each of these conformers with a methyl group, what do you think would be the most favorable position for it? Circle the one H on each structure above that you would replace with a CH3. In general, what happens to the ring C–C–C angles when a ring goes from planar to non-planar? Lecture outline Structures of cycloalkanes — C–C–C angles if planar: 60° 90° 108° 120° actual struct: planar |— slightly |— substantially non-planar —| non-planar —> A compound is strained if it is destabilized by: abnormal bond angles — van der Waals repulsions — eclipsing along σ-bonds — A molecule's strain energy (SE) is the difference between the enthalpy of formation of the compound of interest and that of a hypothetical strain-free compound that has the same atoms connected in exactly the same way. -

MSDS Cyclopentane (1).Pdf
MATERIAL SAFETY DATA SHEET (MSDS) CYCLOPENTANE 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION SUBSTANCE OR PREPATION TRADE NAME Cyclopentane CHEMICAL CLASSIFICATION Cycloparaffin, Naphthene COMPANY/ UNDERTAKING NAME AND Haldia Petrochemicals Limited, ADDRESS PO Box No 12, Haldia Plant PO Durgachak, Dist Midnapore West Bengal, India PIN 721 602 TELEPHONE 091-3224-274384 / 274400 EMERGENCY TELEPHONE NUMBER 091-3224-275916 2. COMPOSTION AND INFORMATION ON INGREDIENTS CHEMICAL CHEMICLAL CONTENT CAS EXPOSURE LIMITS IN AIR NAME FORMULA NUMBER (ppm) ACGIH ACGIH IDLH TLV- TLV- TWA STEL Cyclopentane C5H10 98.20 wt% 287-92-3 600 NA NA 2,2 Dimethyl (CH3)3CCH2CH3 0.28 wt% 75-83-2 500 1000 NA butane n- pentane n- C5H12 1.47 wt% 109-66-0 600 750 NA i- Pentane i- C5H12 0.05 wt% 78-78-4 600 750 NA Total Sulpher S < 1.0 wt. ppm 7704-34-9 NA NA NA 3. HAZARD CLASSIFICATION Highly flammable liquid and vapour. Vapour EMERGENCY OVERVIEW may cause flash fire. Harmful or Fatal is enter lungs and cause damage POTENTIAL HEALTH HAZARDS EYE SKIN INHALATION INGESTION OTHERS ACUTE To cause Not expected Dizziness, Abdominal prolonged or to be harmful headache, pain, significant to internal nausea, diarrhoea, eye irritation organs if Unconsciousness, dizziness, absorbed weakness nausea, sore through the throat skin. CHRONIC Repeated or prolonged contact with skin may cause dermatitis NFPA HAZARD HEALTH FLAMMABILITY REACTIVITY SPECIAL SIGNALS 2 3 0 - HAZCHEM CODE 3YE GHS-Classification Flammable liquids Category 2 Aspiration hazard Category 1 Chronic aquatic toxicity, Category 3 Acute aquatic toxicity, Category 3 Target Organ Systemic Toxicant - Single exposure, Category 3, Inhalation, Central nervous system Document Compiled By Approved By Issue No Rev. -

Cycloalkanes, Cycloalkenes, and Cycloalkynes
CYCLOALKANES, CYCLOALKENES, AND CYCLOALKYNES any important hydrocarbons, known as cycloalkanes, contain rings of carbon atoms linked together by single bonds. The simple cycloalkanes of formula (CH,), make up a particularly important homologous series in which the chemical properties change in a much more dramatic way with increasing n than do those of the acyclic hydrocarbons CH,(CH,),,-,H. The cyclo- alkanes with small rings (n = 3-6) are of special interest in exhibiting chemical properties intermediate between those of alkanes and alkenes. In this chapter we will show how this behavior can be explained in terms of angle strain and steric hindrance, concepts that have been introduced previously and will be used with increasing frequency as we proceed further. We also discuss the conformations of cycloalkanes, especially cyclo- hexane, in detail because of their importance to the chemistry of many kinds of naturally occurring organic compounds. Some attention also will be paid to polycyclic compounds, substances with more than one ring, and to cyclo- alkenes and cycloalkynes. 12-1 NOMENCLATURE AND PHYSICAL PROPERTIES OF CYCLOALKANES The IUPAC system for naming cycloalkanes and cycloalkenes was presented in some detail in Sections 3-2 and 3-3, and you may wish to review that ma- terial before proceeding further. Additional procedures are required for naming 446 12 Cycloalkanes, Cycloalkenes, and Cycloalkynes Table 12-1 Physical Properties of Alkanes and Cycloalkanes Density, Compounds Bp, "C Mp, "C diO,g ml-' propane cyclopropane butane cyclobutane pentane cyclopentane hexane cyclohexane heptane cycloheptane octane cyclooctane nonane cyclononane "At -40". bUnder pressure. polycyclic compounds, which have rings with common carbons, and these will be discussed later in this chapter.