Synergistic Effects of Curcumin and 5-Fluorouracil on The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Luzhou Laojiao Co., Ltd. 2018 Annual Report April 2019
2018 Annual Report of Luzhou Laojiao Co.,Ltd. Luzhou Laojiao Co., Ltd. 2018 Annual Report April 2019 1 2018 Annual Report of Luzhou Laojiao Co.,Ltd. Section I Important Statements, Contents and Definitions The Board of Directors, Board of Supervisors, directors, supervisors and senior management guarantee that the information presented in this report is free of any false records, misleading statements or material omissions, and shall Individually and together be legally liable for truthfulness, accuracy and completeness of its contents. Mr. Liu Miao, responsible person for the Company, Ms. Xie Hong, responsible person for accounting work and Mr. Yan Li, responsible person for the Company’s financial affairs (Accounting Supervisor) have warranted that the financial statements in this report are true and complete. Other directors attended the board meeting to deliberate this report by themselves except the following directors. Name of directors who did not Position of directors who did not Reason for not attending Name of attend the meeting in person attend the meeting in person the meeting in person deputies Liu Miao Chairman of the Board work Lin Feng Shen Caihong Director work Jiang Yuhui Affected by risks, uncertainties and assumptions, the forward-looking statements concerning business objectives and future plans made in this report based on the subjective assumptions and judgments of the future policies and economic conditions may be significantly different from the actual results. Such statements shall not be considered as virtual promises of the Company to investors, and the investors and relevant persons shall maintain adequate risk awareness and shall understand the differences between plans, forecasts and commitments. -

Table of Codes for Each Court of Each Level
Table of Codes for Each Court of Each Level Corresponding Type Chinese Court Region Court Name Administrative Name Code Code Area Supreme People’s Court 最高人民法院 最高法 Higher People's Court of 北京市高级人民 Beijing 京 110000 1 Beijing Municipality 法院 Municipality No. 1 Intermediate People's 北京市第一中级 京 01 2 Court of Beijing Municipality 人民法院 Shijingshan Shijingshan District People’s 北京市石景山区 京 0107 110107 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Haidian District of Haidian District People’s 北京市海淀区人 京 0108 110108 Beijing 1 Court of Beijing Municipality 民法院 Municipality Mentougou Mentougou District People’s 北京市门头沟区 京 0109 110109 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Changping Changping District People’s 北京市昌平区人 京 0114 110114 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Yanqing County People’s 延庆县人民法院 京 0229 110229 Yanqing County 1 Court No. 2 Intermediate People's 北京市第二中级 京 02 2 Court of Beijing Municipality 人民法院 Dongcheng Dongcheng District People’s 北京市东城区人 京 0101 110101 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Xicheng District Xicheng District People’s 北京市西城区人 京 0102 110102 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Fengtai District of Fengtai District People’s 北京市丰台区人 京 0106 110106 Beijing 1 Court of Beijing Municipality 民法院 Municipality 1 Fangshan District Fangshan District People’s 北京市房山区人 京 0111 110111 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Daxing District of Daxing District People’s 北京市大兴区人 京 0115 -
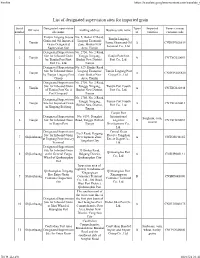
List of Designated Supervision Sites for Imported Grain
Firefox https://translate.googleusercontent.com/translate_f List of designated supervision sites for imported grain Serial Designated supervision Types Imported Venue (venue) Off zone mailing address Business unit name number site name of varieties customs code Tianjin Lingang Jiayue No. 5, Bohai 37 Road, Tianjin Lingang Grain and Oil Imported Lingang Economic 1 Tianjin Jiayue Grain and Oil A CNDGN02S619 Grain Designated Zone, Binhai New Terminal Co., Ltd. Supervision Site Area, Tianjin Designated Supervision No. 2750, No. 2 Road, Site for Inbound Grain Tanggu Xingang, Tianjin Port First 2 Tianjin A CNTXG020051 by Tianjin Port First Binhai New District, Port Co., Ltd. Port Co., Ltd. Tianjin Designated Supervision No. 529, Hunhe Road, Site for Inbound Grain Lingang Economic Tianjin Lingang Port 3 Tianjin A CNDGN02S620 by Tianjin Lingang Port Zone, Binhai New Group Co., Ltd. Group Area, Tianjin Designated Supervision No. 2750, No. 2 Road, Site for Inbound Grain Tanggu Xingang, Tianjin Port Fourth 4 Tianjin A CNTXG020448 of Tianjin Port No. 4 Binhai New District, Port Co., Ltd. Port Company Tianjin No. 2750, No. 2 Road, Designated Supervision Tanggu Xingang, Tianjin Port Fourth 5 Tianjin Site for Imported Grain A CNTXG020413 Binhai New District, Port Co., Ltd. in Xingang Beijiang Tianjin Tianjin Port Designated Supervision No. 6199, Donghai International Sorghum, corn, 6 Tianjin Site for Inbound Grain Road, Tanggu District, Logistics B CNTXG020051 sesame in Tianjin Port Tianjin Development Co., Ltd. Designated Supervision Central Grain No.9 Road, Haigang Site for Inbound Grain Reserve Tangshan 7 Shijiazhuang Development Zone, A CNTGS040165 at Jingtang Port Grocery Direct Depot Co., Tangshan City Terminal Ltd. -

Supplemental Information Nrf1 Is Endowed with a Dominant
Supplemental information Nrf1 is endowed with a dominant tumor-repressing effect onto the Wnt/-Catenin -dependent and -independent signaling networks in the human liver cancer Jiayu Chen1,2,§, Meng Wang1,§, Xufang Ru1,3,§, Yuancai Xiang1,4,§, Yonggang Ren1,5,§, Xiping Liu2, Lu Qiu1,6, and Yiguo Zhang1* 1The Laboratory of Cell Biochemistry and Topogenetic Regulation, College of Bioengineering and Faculty of Sciences, Chongqing University, No. 174 Shazheng Street, Shapingba District, Chongqing 400044, China. 2Department of Biochemistry and Molecular Biology, Zunyi Medical University, No. 6 Xuefu-Xi Road, Xinpu New District, Zunyi 563000, Guizhou, China. 3Department of Neurosurgery, Southwest Hospital, Army (Third Military) Medical University, No. 29 Gaotanyan Street, Shapingba District, Chongqing 400038, China. 4Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Southwest Medical University, No. 1 at the First Section of Xianglin Road, Longmatan District, Luzhou 646000, Sichuan, China. 5Department of Biochemistry, North Sichuan Medical College, No. 55 Dongshun Road, Gaoping District, Nanchong 637000, Sichuan, China. 6School of Life Sciences, Zhengzhou University, No. 100 Kexue Avenue, Zhengzhou 450001, Henan, China. §Contributed equally to this work. *Correspondence should be addressed to Yiguo Zhang (Email: [email protected], or [email protected]) 1. Supplemental materials and methods 1.1 The TOPflash Luciferase reporter assay to measure β-catenin/Tcf-driven transcriptional activity. Human 293T cells (2×104) were seeded in each well of a 48-vial plate and allowed for growth to 70% confluence. The cells were co-transfected with 100 ng of the firefly luciferase reporter called TOPflash (driven by the consensus β-catenin/Tcf4-binding site) or its mutant control plasmid called FOPflash, together with 5 ng of Renilla luciferase reporter (pRL-CMV), plus 10 pmol of indicated small interference RNA targeting for Nrf1 (i.e., siNrf1) or a scrambled negative control RNA (i.e. -

Luzhou Laojiao Co., Ltd. 2019 Annual Report April 2020
2019 Annual Report of Luzhou Laojiao Co., Ltd. Luzhou Laojiao Co., Ltd. 2019 Annual Report April 2020 1 2019 Annual Report of Luzhou Laojiao Co., Ltd. Section I Important Statements, Contents and Definitions The Board of Directors, Board of Supervisors, directors, supervisors and senior management guarantee that the information presented in this report is free of any false records, misleading statements or material omissions, and shall individually and together be legally liable for truthfulness, accuracy and completeness of its contents. Mr. Liu Miao, responsible person for the Company, Ms. Xie Hong, responsible person for accounting and Ms. Yan Li, responsible person for the Company’s financial affairs (Accounting Supervisor) have warranted that the financial statements in this report are true and complete. Other directors attended the board meeting to deliberate this report by themselves except the following directors. Position of directors who Name of directors who did not Reason for not attending did not attend the meeting Name of deputies attend the meeting in person the meeting in person in person Lin Feng Director Work Liu Miao Affected by risks, uncertainties and assumptions, the forward-looking statements concerning business objectives and future plans made in this report based on the subjective assumptions and judgments of the future policies and economic conditions may be significantly different from the actual results. Such statements shall not be considered as virtual promises of the Company to investors, and the investors and relevant persons shall maintain adequate risk awareness and shall understand the differences between plans, forecasts and commitments. In the annual report, the potential risks in the operation of the Company have been disclosed. -

Global Offering
瀘州市商業銀行股份有限公司 Luzhou City Commercial Bank Co., Ltd. (A joint stock company incorporated in the People's Republic of China with limited liability) Stock Code: 1983 Global offerinG Sole Sponsor Joint Global Coordinators, Joint Bookrunners and Joint Lead Managers Joint Lead Managers IMPORTANT IMPORTANT: If you are in doubt about any information contained in this prospectus you should obtain independent professional advice. Luzhou City Commercial Bank Co., Ltd.* 瀘州市商業銀行股份有限公司* (A joint stock company incorporated in the People’s Republic of China with limited liability) GLOBAL OFFERING Number of Offer Shares in the : 545,740,000 H Shares (subject to the Global Offering Over-allotment Option) Number of International Offer Shares : 491,166,000 H Shares (subject to adjustment and the Over-allotment Option) Number of Hong Kong Offer Shares : 54,574,000 H Shares (subject to adjustment) Maximum Offer Price : HK$3.40 per H Share, plus brokerage of 1%, SFC transaction levy of 0.0027% and Hong Kong Stock Exchange trading fee of 0.005% (payable in full on application in Hong Kong dollars, subject to refund) Nominal value : RMB1.00 per H Share Stock code : 1983 Sole Sponsor Joint Global Coordinators, Joint Bookrunners and Joint Lead Managers Joint Lead Managers Hong Kong Exchanges and Clearing Limited, The Stock Exchange of Hong Kong Limited and Hong Kong Securities Clearing Company Limited take no responsibility for the contents of this prospectus, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this prospectus. -
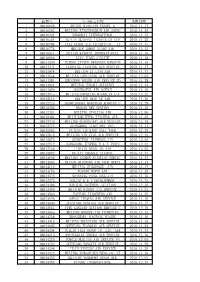
航协号 公司英文名称 1 08010026 Shishi Qiaolian Travel & 2016.11.17 2 08010262 Beijing Xingzhongbin Air Agenc 2016.1
航协号 公司英文名称 到账日期 1 08010026 SHISHI QIAOLIAN TRAVEL & 2016.11.17 2 08010262 BEIJING XINGZHONGBIN AIR AGENC 2016.11.29 3 08010424 SHANGHAI INTERNATIONAL 2016.11.25 4 08010564 GUILIN GUIKANG TICKETS CO LTD 2016.11.25 5 08010704 CIXI XUNDA AIR TICKETS CO., LT 2016.11.15 6 08010774 BEIJING ZHENG XIANG AIR 2016.12.21 7 08010811 FUJIAN KANGTAI INTERNATIONAL 2016.11.16 8 08010833 LIAN JIANG AIRLINE 2016.11.18 9 08011290 FUZHOU JINYUN HANGKONG HANGYUN 2016.11.16 10 08011441 LIAONING JIANTONG AIR SERVICE 2016.11.17 11 08011474 BEIJING ZI LANG AIR 2016.11.22 12 08011544 BEIJING LANYUXING AIR SERVICE 2016.12.01 13 08011581 SHENZHEN YOUSHI AIR SERVICE CO 2016.11.28 14 08011824 BEIJING ZHAORI AVIATION 2016.11.14 15 08011850 SUCCESSFUL AIR AGENCY 2016.11.16 16 08012045 BEIJING HANGTIAN MIANYUAN AIR 2016.12.22 17 08012115 BEIJING QING YE AIR 2016.11.30 18 08012325 QINHUANGDAO HONGYUAN KONGYUN C 2016.11.29 19 08012336 HANDAN NEW CENTURY 2016.11.15 20 08012351 BEIJING JINGJIAO AIR 2016.12.06 21 08012384 BEIJINGRUIFENG XINCHENG AIR 2016.11.25 22 08012443 BEIJING GENERALWAY AIR SERVICE 2016.12.15 23 08012572 GUANGZHOU JIAOYIHUI INTL 2016.11.17 24 08012583 FUJIAN JINJIANG ANLI TOUR 2016.11.28 25 08012642 BEIJING YIN YING AIR SERVICE 2016.12.19 26 08012675 GUANGZHOU TIANWANG AIR 2016.12.05 27 08012712 GUANGDONG JINPENG E & T INDUS 2016.12.22 28 08012756 TIANJIN HONGLIAN AIR 2016.12.02 29 08012992 FUJIAN CHANGLE XIANGYU 2016.11.17 30 08013036 BEIJING ANZHEN AVIATION SERVIC 2016.11.23 31 08013062 HAINAN HAICHENG AIR TOUR SERVI 2016.11.17 32 08013110 BEIJING ZIGUANGGE -

中國內地指定醫院列表 出版日期: 2019 年 7 月 1 日 Designated Hospital List in Mainland China Published Date: 1 Jul 2019
中國內地指定醫院列表 出版日期: 2019 年 7 月 1 日 Designated Hospital List in Mainland China Published Date: 1 Jul 2019 省 / 自治區 / 直轄市 醫院 地址 電話號碼 Provinces / 城市/City Autonomous Hospital Address Tel. No. Regions / Municipalities 中國人民解放軍第二炮兵總醫院 (第 262 醫院) 北京 北京 西城區新街口外大街 16 號 The Second Artillery General Hospital of Chinese 10-66343055 Beijing Beijing 16 Xinjiekou Outer Street, Xicheng District People’s Liberation Army 中國人民解放軍總醫院 (第 301 醫院) 北京 北京 海澱區復興路 28 號 The General Hospital of Chinese People's Liberation 10-82266699 Beijing Beijing 28 Fuxing Road, Haidian District Army 北京 北京 中國人民解放軍第 302 醫院 豐台區西四環中路 100 號 10-66933129 Beijing Beijing 302 Military Hospital of China 100 West No.4 Ring Road Middle, Fengtai District 中國人民解放軍總醫院第一附屬醫院 (中國人民解 北京 北京 海定區阜成路 51 號 放軍 304 醫院) 10-66867304 Beijing Beijing 51 Fucheng Road, Haidian District PLA No.304 Hospital 北京 北京 中國人民解放軍第 305 醫院 西城區文津街甲 13 號 10-66004120 Beijing Beijing PLA No.305 Hospital 13 Wenjin Street, Xicheng District 北京 北京 中國人民解放軍第 306 醫院 朝陽區安翔北里 9 號 10-66356729 Beijing Beijing The 306th Hospital of PLA 9 Anxiang North Road, Chaoyang District 中國人民解放軍第 307 醫院 北京 北京 豐台區東大街 8 號 The 307th Hospital of Chinese People’s Liberation 10-66947114 Beijing Beijing 8 East Street, Fengtai District Army 中國人民解放軍第 309 醫院 北京 北京 海澱區黑山扈路甲 17 號 The 309th Hospital of Chinese People’s Liberation 10-66775961 Beijing Beijing 17 Heishanhu Road, Haidian District Army 中國人民解放軍第 466 醫院 (空軍航空醫學研究所 北京 北京 海澱區北窪路北口 附屬醫院) 10-81988888 Beijing Beijing Beiwa Road North, Haidian District PLA No.466 Hospital 北京 北京 中國人民解放軍海軍總醫院 (海軍總醫院) 海澱區阜成路 6 號 10-66958114 Beijing Beijing PLA Naval General Hospital 6 Fucheng Road, Haidian District 北京 北京 中國人民解放軍空軍總醫院 (空軍總醫院) 海澱區阜成路 30 號 10-68410099 Beijing Beijing Air Force General Hospital, PLA 30 Fucheng Road, Haidian District 中華人民共和國北京市昌平區生命園路 1 號 北京 北京 北京大學國際醫院 Yard No.1, Life Science Park, Changping District, Beijing, 10-69006666 Beijing Beijing Peking University International Hospital China, 東城區南門倉 5 號(西院) 5 Nanmencang, Dongcheng District (West Campus) 北京 北京 北京軍區總醫院 10-66721629 Beijing Beijing PLA. -

Research Article Nrf1 Is Endowed with a Dominant Tumor-Repressing
Hindawi Oxidative Medicine and Cellular Longevity Volume 2020, Article ID 5138539, 28 pages https://doi.org/10.1155/2020/5138539 Research Article Nrf1 Is Endowed with a Dominant Tumor-Repressing Effect onto the Wnt/β-Catenin-Dependent and Wnt/β-Catenin-Independent Signaling Networks in the Human Liver Cancer Jiayu Chen,1,2 Meng Wang,1 Yuancai Xiang,1,3 Xufang Ru,1,4 Yonggang Ren,1,5 Xiping Liu,2 Lu Qiu,1,6 and Yiguo Zhang 1 1The Laboratory of Cell Biochemistry and Topogenetic Regulation, College of Bioengineering and Faculty of Sciences, Chongqing University, No. 174 Shazheng Street, Shapingba District, Chongqing 400044, China 2Department of Biochemistry and Molecular Biology, Zunyi Medical University, No. 6 Xuefu-Xi Road, Xinpu New District, Zunyi, 563000 Guizhou, China 3Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Southwest Medical University, No. 1 at the First Section of Xianglin Road, Longmatan District, Luzhou, 646000 Sichuan, China 4Department of Neurosurgery, Southwest Hospital, Army (Third Military) Medical University, No. 29 Gaotanyan Street, Shapingba District, Chongqing 400038, China 5Department of Biochemistry, North Sichuan Medical College, No. 55 Dongshun Road, Gaoping District, Nanchong, 637000 Sichuan, China 6School of Life Sciences, Zhengzhou University, No. 100 Kexue Avenue, Zhengzhou, 450001 Henan, China Correspondence should be addressed to Yiguo Zhang; [email protected] Received 7 January 2020; Accepted 20 February 2020; Published 23 March 2020 Academic Editor: Manuela Curcio Copyright © 2020 Jiayu Chen et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. -

Biomechanical Testing of Three Coracoclavicular Ligament Reconstruction Techniques with a 3D Printing Navigation Template for Clavicle-Coracoid Drilling
1121 Original Article Page 1 of 12 Biomechanical testing of three coracoclavicular ligament reconstruction techniques with a 3D printing navigation template for clavicle-coracoid drilling Ji Qi1,2,3,4#^, Shijie Fu5,6,7,8#, Ruiyue Ping1,3#^, Kai Wu9#, Ziyu Feng10, Yanxiao Xu11, Xiaoguang Guo5, Dingkun Lin1,3, Lei Zhang5,6,7,8^ 1Department of Orthopaedics, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China; 2Wang Jing Hospital of China Academy of Chinese Medical Sciences, Beijing, China; 3The Second Clinical College of Guangzhou University of Chinese Medicine, Guangzhou, China; 4Postdoctoral Research Station, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China; 5Department of Orthopaedics, Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University, Luzhou, China; 6Center for Orthopaedic Diseases Research, Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University, Luzhou, China; 7Clinical Base of Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University, Guangdong Province Medical 3D Printing Application Transformation Engineering Technology Research Center, Luzhou, China; 8Expert Workstation in Luzhou, Luzhou, China; 9The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China; 10Southern Medical University, Guangzhou, China; 11Suzhou Yongding Hospital, Suzhou, China Contributions: (I) Conception and design: J Qi, S Fu, R Ping, K Wu, D Lin, L Zhang; (II) Administrative support: D Lin, L Zhang; (III) Provision of study materials or patients: Z Feng, Y Xu, X Guo; (IV) Collection and assembly of data: J Qi, S Fu, R Ping, K Wu, Y Xu, X Guo; (V) Data analysis and interpretation: Z Feng, X Guo; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. #These authors contributed equally to this work. -

Agricultural Production Conditions for Establishing a Commodity
2019 2nd International Workshop on Advances in Social Sciences (IWASS 2019) Agricultural Production Conditions for Establishing a Commodity Trading Market under the Belt and Road Initiative --An Empirical Analysis of VAR Model Based on Statistical Data of South Sichuan Port-vicinity Pilot Free Trade Zone Gengzhi Liu 1,2 1. School of Marxism, Renmin University of China, Beijing, China, 100872; 2. Bureau for Economic Policy Analysis, South Sichuan Port-vicinity Area of China (Sichuan) Pilot Free Trade Zone, Luzhou, Sichan, China, 646000 Keywords: “the belt and road” initiative; Commodity trading market; Agricultural production conditions; South sichuan port-vicinity pilot free trade zone; Var model Abstract: “The Belt and Road” initiative and the “Pilot Free Trade Zone” are major strategic measures for national governance and deepening reform and opening up in the new era, and actively linking them will help enhance trade liberalization and openness. Because of the large volume and amount of transactions, commodity trading markets play an important role in activating the “China-EU railway” of the Belt and Road initiative and revitalize the “stock” of the open economy and enhance the “trade volume” of trade. Based on the investigation and statistical analysis of the micro-basis of the establishment of the commodity trading market in the South Sichuan Port-vicinity Pilot Free Trade Zone, it is found that the output value and sown area of important commodities have declined to varying degrees in 2018, which is extremely unfavorable for the selection of trading varieties and the high quality supply of commodity sources in the forthcoming commodity trading market. -
![Directors, Supervisors and Parties Involved in the [Redacted]](https://docslib.b-cdn.net/cover/0425/directors-supervisors-and-parties-involved-in-the-redacted-4650425.webp)
Directors, Supervisors and Parties Involved in the [Redacted]
THIS DOCUMENT IS IN DRAFT FORM, INCOMPLETE AND SUBJECT TO CHANGE AND THE INFORMATION MUST BE READ IN CONJUNCTION WITH THE SECTION HEADED “WARNING” ON THE COVER OF THIS DOCUMENT. DIRECTORS, SUPERVISORS AND PARTIES INVOLVED IN THE [REDACTED] Name Residential Address Nationality Executive Directors Mr. YOU Jiang (游江) No. 8, 4/F, Unit 3, Building 4 Chinese (Chairman) No. 38 Beishuncheng Street Jinjiang District Chengdu Sichuan Province the PRC Mr. XU Xianzhong (徐先忠) No. 23, Building 2 Chinese No. 18 Datong Road Longmatan District Luzhou Sichuan Province the PRC Mr. LIU Shirong (劉仕榮) No. 1, Building 3 Chinese No. 5 Jinzhu Lane Jiangyang District Luzhou Sichuan Province the PRC Non-executive Directors Ms. XU Yan (徐燕) No. 66, 17/F, Unit 2 Chinese Peninsula No. 2 Binjiang Road Jiangyang District Luzhou Sichuan Province the PRC Mr. XIONG Guoming (熊國銘) No. 2, Unit 1, Building 12 Chinese Binjiang Community Committee Nancheng Street Jiangyang District Luzhou Sichuan Province the PRC Mr. LIU Qi (劉奇) No. 9, Unit 2, Building 8 Chinese No. 9 Danqing Road Jiangyang District Luzhou Sichuan Province the PRC Mr. DAI Zhiwei (代志偉) 19-1-3, Baizhuyuan, Erzutuan Chinese East Danxia Road Jiangyang District Luzhou Sichuan Province the PRC 73 THIS DOCUMENT IS IN DRAFT FORM, INCOMPLETE AND SUBJECT TO CHANGE AND THE INFORMATION MUST BE READ IN CONJUNCTION WITH THE SECTION HEADED “WARNING” ON THE COVER OF THIS DOCUMENT. DIRECTORS, SUPERVISORS AND PARTIES INVOLVED IN THE [REDACTED] Name Residential Address Nationality Independent Non-executive Directors Mr. LIU Xiaoyu (劉小渝) No. 1, Unit 2, Building 2 Chinese No. 48 Longtouguan Road Jiangyang District Luzhou Sichuan Province the PRC Mr.