Functional Domains for Assembly of Histones H3 and H4 Into the Chromatin of Xenopus Embryos
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Topography of the Histone Octamer Surface: Repeating Structural Motifs Utilized in the Docking of Nucleosomal
Proc. Natl. Acad. Sci. USA Vol. 90, pp. 10489-10493, November 1993 Biochemistry Topography of the histone octamer surface: Repeating structural motifs utilized in the docking of nucleosomal DNA (histone fold/helix-strand-helix motif/parallel fi bridge/binary DNA binding sites/nucleosome) GINA ARENTS* AND EVANGELOS N. MOUDRIANAKIS*t *Department of Biology, The Johns Hopkins University, Baltimore, MD 21218; and tDepartment of Biology, University of Athens, Athens, Greece Communicated by Christian B. Anfinsen, August 5, 1993 ABSTRACT The histone octamer core of the nucleosome is interactions. The model offers strong predictive criteria for a protein superhelix offour spirally arrayed histone dimers. The structural and genetic biology. cylindrical face of this superhelix is marked by intradimer and interdimer pseudodyad axes, which derive from the nature ofthe METHODS histone fold. The histone fold appears as the result of a tandem, parallel duplication of the "helix-strand-helix" motif. This The determination of the structure of the histone octamer at motif, by its occurrence in the four dimers, gives rise torepetitive 3.1 A has been described (3). The overall shape and volume structural elements-i.e., the "parallel 13 bridges" and the of this tripartite structure is in agreement with the results of "paired ends of helix I" motifs. A preponderance of positive three independent studies based on differing methodolo- charges on the surface of the octamer appears as a left-handed gies-i.e., x-ray diffraction, neutron diffraction, and electron spiral situated at the expected path of the DNA. We have microscopic image reconstruction (4-6). Furthermore, the matched a subset of DNA pseudodyads with the octamer identification of the histone fold, a tertiary structure motif of pseudodyads and thus have built a model of the nucleosome. -

Recurrent Evolution of DNA-Binding Motifs in the Drosophila Centromeric Histone
Recurrent evolution of DNA-binding motifs in the Drosophila centromeric histone Harmit S. Malik*, Danielle Vermaak*, and Steven Henikoff*†‡ †Howard Hughes Medical Institute, *Basic Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, WA 98109 Communicated by Stanley M. Gartler, University of Washington, Seattle, WA, December 12, 2001 (received for review October 29, 2001) All eukaryotes contain centromere-specific histone H3 variants being one of the most evolutionarily constrained classes of (CenH3s), which replace H3 in centromeric chromatin. We have eukaryotic proteins. Second, CenH3s are evolving adaptively, previously documented the adaptive evolution of the Drosophila presumably in concert with centromeres, which consist of the CenH3 (Cid) in comparisons of Drosophila melanogaster and Dro- most rapidly evolving DNA in the genome. sophila simulans, a divergence of Ϸ2.5 million years. We have Chromosomes (and their centromeres) may compete at mei- proposed that rapidly changing centromeric DNA may be driving osis I for inclusion into the oocyte (11). A centromeric satellite CenH3’s altered DNA-binding specificity. Here, we compare Cid expansion could bias centromeric strength, leading to preferred sequences from a phylogenetically broader group of Drosophila inclusion in the oocyte, but also to increased nondisjunction. A species to suggest that Cid has been evolving adaptively for at least subsequent alteration of CenH3’s DNA-binding preferences 25 million years. Our analysis also reveals conserved blocks not could restore parity among different chromosomes (2), thereby only in the histone-fold domain but also in the N-terminal tail. In alleviating the deleterious consequences of satellite drive. Suc- several lineages, the N-terminal tail of Cid is characterized by cessive rounds of satellite and CenH3 drive are detected as an subgroup-specific oligopeptide expansions. -
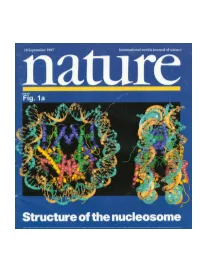
Crystal Structure of the Nucleosome Core Particle at 2.8Å Resolution Karolin Luger, Armin W
Crystal structure of the nucleosome core particle at 2.8Å resolution Karolin Luger, Armin W. Mader, Robin K. Richmond, David R Sargent & Timothy J. Richmond Institut fur Molekularbiologie und Biophysik ETHZ, ETH-Hönggerberg, CH-8093 Zürich, Switzerland Nature, Vol 389, pages 251-260 The X-ray crystal structure of the nucleosome core particle of chromatin shows in atomic detail how the histone protein octamer is assembled and how 146 base pairs of DNA are organized into a superhelix around it. Both histone/histone and histone/DNA interactions depend on the histone fold domains and additional, well ordered structure elements extending from this motif. Histone amino-terminal tails pass over and between the gyros of the DNA superhelix to contact neighboring particles. The lack of uniformity between multiple histone/DNA-binding sites causes the DNA to deviate from ideal superhelix geometry. DNA in chromatin is organized in arrays of nucleosomes (1). Two copies of each histone protein, H2A, H2B, H3 and H4, are assembled into an octamer that has 145-147 base pairs (bp) of DNA wrapped around it to form a nucleosome core (of relative molecular mass 206K). This highly conserved nucleoprotein complex occurs essentially every 200 ± 40 bp throughout all eukaryotic genomes (2). The repeating nucleosome cores further assemble into higher-order structures which are stabilized by the linker histone H1 and these compact linear DNA overall by a factor of 30-40. The nucleosome (nucleosome core, linker DNA and H1) thereby shapes the DNA molecule both at the atomic level through DNA bending, and on the much larger scale of genes by forming higher- order helices (3). -

Containing Protein That Replaces TAF6 in Drosophila SAGA Is Required for SAGA-Dependent Gene Expression
Downloaded from genesdev.cshlp.org on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press RESEARCH COMMUNICATION shown previously that Drosophila SAGA (dSAGA) in- A novel histone fold domain- cludes the orthologs of most components of the yeast containing protein that replaces SAGA (ySAGA) complex (Supplemental Table S1; Kusch et al. 2003; Muratoglu et al. 2003; Guelman et al. 2006; TAF6 in Drosophila SAGA is Kurshakova et al. 2007; Weake et al. 2008). In addition, required for SAGA-dependent dSAGA contains subunits that are unique to the fly com- plex, such as the WD repeat-containing protein WDA gene expression (Guelman et al. 2006). SAGA is essential for development in multicellular Vikki M. Weake,1 Selene K. Swanson,1 organisms, and Gcn5 is required for viability in both Arcady Mushegian,1,2 Laurence Florens,1 mice and Drosophila (Xu et al. 2000; Carre et al. 2005). Michael P. Washburn,1,3 Susan M. Abmayr,1,4 Furthermore, mutations that disrupt the HAT activity of and Jerry L. Workman1,5 dSAGA, such as ada2b and wda, result in lethality in flies (Qi et al. 2004; Pankotai et al. 2005; Guelman et al. 2006). 1Stowers Institute for Medical Research, Kansas City, Missouri Moreover, mutations that specifically affect the ubiquitin 64110, USA; 2Department of Microbiology, Molecular Genetics protease activity of dSAGA are lethal, and result in and Immunology, University of Kansas Medical Center, Kansas defects in axon targeting in the larval eye–brain complex City, Kansas 66160, USA; 3Department of Pathology and (Weake et al. 2008). To characterize dSAGA more fully, Laboratory Medicine, University of Kansas Medical Center, we sought to identify orthologs of all ySAGA compo- Kansas City, Kansas 66160, USA; 4Department of Anatomy and nents, as there are subunits present in the ySAGA and Cell Biology, University of Kansas Medical Center, Kansas City, human SAGA complexes for which orthologs have not Kansas 66160, USA yet been identified in flies (Rodriguez-Navarro 2009). -

Histone Variants — Ancient Wrap Artists of the Epigenome
REVIEWS CHROMATIN DYNAMICS Histone variants — ancient wrap artists of the epigenome Paul B. Talbert and Steven Henikoff Abstract | Histones wrap DNA to form nucleosome particles that compact eukaryotic genomes. Variant histones have evolved crucial roles in chromosome segregation, transcriptional regulation, DNA repair, sperm packaging and other processes. ‘Universal’ histone variants emerged early in eukaryotic evolution and were later displaced for bulk packaging roles by the canonical histones (H2A, H2B, H3 and H4), the synthesis of which is coupled to DNA replication. Further specializations of histone variants have evolved in some lineages to perform additional tasks. Differences among histone variants in their stability, DNA wrapping, specialized domains that regulate access to DNA, and post-translational modifications, underlie the diverse functions that histones have acquired in evolution. Histone chaperone Nearly all eukaryotes wrap their DNA around histones to In this Review, we consider the diversity and evolution of An escort protein that form nucleosomes that compact the genome while still core histone variants, from archaeal ancestors to univer- performs a transfer reaction on allowing access for active processes such as trans cription, sal eukaryotic variants to lineage-specific variants and a histone, such as deposition replication and DNA repair. Each nucleosome core variant-specific post-translational modifications (PTMs). onto DNA, eviction from DNA, transfer to another chaperone particle comprises ~ 147 bp of DNA wrapped in 1.7 turns We summarize their diverse and dynamic interactions as or enzyme, or storage for later around a protein octamer of 2 molecules of each of the 4 ‘wrap artists’ of DNA and discuss recent developments use. -

Histone H4 and the Maintenance of Genome Integrity
Downloaded from genesdev.cshlp.org on September 30, 2021 - Published by Cold Spring Harbor Laboratory Press Histone H4 and the maintenance of genome integrity Paul C. Megee, 1 Brian A. Morgan, 2 and M. Mitchell Smith 3 Department of Microbiology, University of Virginia School of Medicine Charlottesville, Virginia 22908 USA The normal progression of Saccharomyces cerevisiae through nuclear division requires the function of the amino-terminal domain of histone H4. Mutations that delete the domain, or alter 4 conserved lysine residues within the domain, cause a marked delay during the G2 +M phases of the cell cycle. Site-directed mutagenesis of single and multiple lysine residues failed to map this phenotype to any particular site; the defect was only observed when all four lysines were mutated. Starting with a quadruple lysine-to-glutamine substitution allele, the insertion of a tripeptide containing a single extra lysine residue suppressed the G2+M cell cycle defect. Thus, the amino-terminal domain of histone H4 has novel genetic functions that depend on the presence of lysine per se, and not a specific primary peptide sequence. To determine the nature of this function, we examined H4 mutants that were also defective for G2/M checkpoint pathways. Disruption of the mitotic spindle checkpoint pathway had no effect on the phenotype of the histone amino-terminal domain mutant. However, disruption of RADg, which is part of the pathway that monitors DNA integrity, caused precocious progression of the H4 mutant through nuclear division and increased cell death. These results indicate that the lysine-dependent function of histone H4 is required for the maintenance of genome integrity, and that DNA damage resulting from the loss of this function activates the RAD9-dependent G2/M checkpoint pathway. -

Commentary the Histone Fold: Evolutionary Questions V
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 11328-11330, December 1995 Commentary The histone fold: Evolutionary questions V. Ramakrishnan Department of Biochemistry, University of Utah School of Medicine, Salt Lake City, UT 84132 While histones as a class of protein asso- of each dimer pair had been suggested by central helix in the histone fold arose as a ciated with DNA have been known for NMR studies (7, 8). result of a fusion between helices from over a hundred years, our current ideas of In a recent issue of the Proceedings (9), each HSH motif. Since the basic structural how histones associate with DNA began Arents and Moudrianakis use the struc- unit is half a fold, this lends support to the with the discovery of the nucleosome as a ture of the core histones to investigate the idea of a gene-doubling in the histones, basic structural unit of chromatin (1, 2). In chemical and structural nature of the his- although it is clearly present in all the the nucleosome, two left-handed super- tone fold and offer arguments for a com- histones and not just H2A as suggested by helical turns of DNA wrap around an mon origin for the core histones. In a sequence comparison studies. Moreover, octameric histone core consisting of two related paper (10), it is shown that the this suggests that the gene-doubling event copies of each of the four core histones- histone fold occurs in a variety of different occurred before the differentiation of the H2A, H2B, H3, and H4. proteins. Taken together, these results core histones into the four types. -

The Histone Fold
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 11170-11174, November 1995 Biochemistry The histone fold: A ubiqu'itous architectural motif utilized in DNA compaction and protein dimerization (paired-element motif/archaeal histones/centromeric CENP-A/transcription/evolution) GINA ARENTS* AND EVANGELOS N. MOUDRIANAKIS*t *Department of Biology, The Johns Hopkins University, Baltimore, MD 21218; and tBiology Department, University of Athens, Athens, Greece Communicated by Hamilton 0. Smith, Johns Hopkins School of Medicine, Baltimore, MD, August 21, 1995 (received for review July 6, 1995) ABSTRACT The histones of all eukaryotes show only a histone fold regions of the four histone chains will be referred low degree of primary structure homology, but our earlier to as FH2A, FH2B, FH3, and FH4. All comparisons between crystallographic results defined a three-dimensional struc- histone structures were performed using the program ALIGN, tural motif, the histone fold, common to all core histones. We kindly made available to us by Mario Amzel. ALIGN calculates now examine the specific architectural patterns within the fold the best fit and rms deviation between sets of equivalent a and analyze the nature of the amino acid residues within its carbon coordinates. The histone coordinates employed in the functional segments. The histone fold emerges as a funda- calculation are taken from the 3.1-A, partially refined (R = mental protein dimerization motifwhile the differentiations of 26%), histone octamer structure (1). In performing the cal- the tips of the histone dimers appear to provide the rules of culations, allowance was made for a single-site deletion in the core octamer assembly and the basis for nucleosome regula- loop region ofH4 that follows helix I. -
H2A Histone-Fold and DNA Elements in Nucleosome Activate SWR1
1 2 3 4 5 H2A histone-fold and DNA elements in nucleosome 6 activate SWR1-mediated H2A.Z replacement 7 8 9 10 11 12 13 14 15 16 Anand Ranjan1,*, Feng Wang2, Gaku Mizuguchi1, Debbie Wei2, Yingzi 17 Huang2, and Carl Wu1,2,* 18 19 20 21 22 23 24 1Janelia Research Campus, Howard Hughes Medical Institute, Ashburn, United 25 States 26 2Laboratory of Biochemistry and Molecular Biology, National Cancer Institute, 27 National Institutes of Health, Bethesda, United States 28 29 *Correspondence: [email protected] (A.R.), [email protected] (C.W.) 30 Ranjan et al. 2015 31 Abstract 32 The histone variant H2A.Z is a universal mark of gene promoters, enhancers and 33 regulatory elements in eukaryotic chromatin. The chromatin remodeler SWR1 34 mediates site-specific incorporation of H2A.Z by a multi-step histone replacement 35 reaction, evicting histone H2A-H2B from the canonical nucleosome and 36 depositing the H2A.Z-H2B dimer. Binding of both substrates—the canonical 37 nucleosome and the H2A.Z-H2B dimer, is essential for activation of SWR1. We 38 found that SWR1 primarily recognizes key residues within the α2 helix in the 39 histone-fold of nucleosomal histone H2A, a region not previously known to 40 influence remodeler activity. Moreover, SWR1 interacts preferentially with 41 nucleosomal DNA at superhelix location 2 on the nucleosome face distal to its 42 linker-binding site. Our findings provide new molecular insights on recognition of 43 the canonical nucleosome by a chromatin remodeler, and have implications for 44 ATP-driven mechanisms of histone eviction and deposition. -
Histone Variants in Archaea and the Evolution of Combinatorial Chromatin Complexity
Histone variants in archaea and the evolution of combinatorial chromatin complexity Kathryn M. Stevensa,b, Jacob B. Swadlinga,b, Antoine Hochera,b, Corinna Bangc,d, Simonetta Gribaldoe, Ruth A. Schmitzc, and Tobias Warneckea,b,1 aMolecular Systems Group, Quantitative Biology Section, Medical Research Council London Institute of Medical Sciences, London W12 0NN, United Kingdom; bInstitute of Clinical Sciences, Faculty of Medicine, Imperial College London, London W12 0NN, United Kingdom; cInstitute for General Microbiology, University of Kiel, 24118 Kiel, Germany; dInstitute of Clinical Molecular Biology, University of Kiel, 24105 Kiel, Germany; and eDepartment of Microbiology, Unit “Evolutionary Biology of the Microbial Cell,” Institut Pasteur, 75015 Paris, France Edited by W. Ford Doolittle, Dalhousie University, Halifax, NS, Canada, and approved October 28, 2020 (received for review April 14, 2020) Nucleosomes in eukaryotes act as platforms for the dynamic inte- additional histone dimers can be taggedontothistetramertoyield gration of epigenetic information. Posttranslational modifications oligomers of increasing length that wrap correspondingly more DNA are reversibly added or removed and core histones exchanged for (3, 6–9). Almost all archaeal histones lack tails and PTMs have yet to paralogous variants, in concert with changing demands on tran- be reported. Many archaea do, however, encode multiple histone scription and genome accessibility. Histones are also common in paralogs (8, 10) that can flexibly homo- and heterodimerize in -

Acetylation of H2AZ Lys 14 Is Associated with Genome-Wide Gene Activity in Yeast
Downloaded from genesdev.cshlp.org on September 27, 2021 - Published by Cold Spring Harbor Laboratory Press Acetylation of H2AZ Lys 14 is associated with genome-wide gene activity in yeast Catherine B. Millar,1 Feng Xu,1 Kangling Zhang,2 and Michael Grunstein1,3 1Department of Biological Chemistry, Geffen School of Medicine and the Molecular Biology Institute, University of California, Los Angeles, California 90095, USA; 2Mass Spectrometry Facility, Department of Chemistry, University of California, Riverside, California 92521, USA Histone variants and their post-translational modifications help regulate chromosomal functions. Htz1 is an evolutionarily conserved H2A variant found at several promoters in the yeast Saccharomyces cerevisiae.In this study, we undertook a genome-wide analysis of Htz1 and its modifications in yeast. Using mass spectrometric analysis, we determined that Htz1 is acetylated at Lys 3, Lys 8, Lys 10, and Lys 14 within its N-terminal tail, with K14 being the most abundant acetylated site. ChIP and microarray analysis were then used to compare the location of Htz1-K14 acetylation to that of Htz1 genome-wide. The data presented here demonstrate that while Htz1 is associated preferentially with the promoters of repressed genes, K14 acetylation is enriched at the promoters of active genes, and requires two known histone acetyltransferases, Gcn5 and Esa1. In support of our genome-wide analysis, we found that the acetylatable lysines of Htz1 are required for its full deposition during nucleosome reassembly upon repression of PHO5. Since the majority of Htz1 acetylation is seen at active promoters, where nucleosomes are known to be disassembled, our data argue for a dynamic process in which reassembly of Htz1 is regulated by its acetylation at promoters during transcription. -

Structural Characterization of Histone H2A Variants
27_Symp69_Chakrava_p.227_234 4/21/05 9:32 AM Page 227 Structural Characterization of Histone H2A Variants S. CHAKRAVARTHY,* Y. BAO,* V.A. ROBERTS,† D. TREMETHICK,‡ AND K. LUGER* *Department of Biochemistry and Molecular Biology, Colorado State University, Fort Collins, Colorado 80523-1870; †Department of Molecular Biology, The Scripps Research Institute, La Jolla, California 92037; ‡The John Curtin School of Medical Research, The Australian National University, Australian Capital University, Canberra ACT, 2601 Australia Eukaryotic DNA associates with an equal amount of pendent processes. Much progress has been made in this protein to form chromatin, the fundamental unit of which direction in the past few years. is the nucleosome core particle (NCP). An NCP consists Substitution of one or more of the core histones with of two copies each of the four core histones H2A, H2B, the corresponding histone variants has the potential to ex- H3, and H4. This histone octamer binds 147 base pairs of ert considerable influence on the structure and function of DNA around its outer surface in 1.65 tight superhelical chromatin. Histone variants are distinct nonallelic forms turns (Fig. 1A) (Luger et al. 1997; Richmond and Davey of conventional, major-type histones that form the bulk of 2003). Linker histones and other nonhistone proteins pro- nucleosomes during replication and whose synthesis is mote or stabilize the folding of nucleosomal arrays into tightly coupled to S phase. Histone variants are charac- superstructures of increasing complexity and largely un- terized by a completely different expression pattern that known architecture (Hansen 2002). Covalent modifica- is not restricted to S phase.