Heat Engines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Physics 170 - Thermodynamic Lecture 40
Physics 170 - Thermodynamic Lecture 40 ! The second law of thermodynamic 1 The Second Law of Thermodynamics and Entropy There are several diferent forms of the second law of thermodynamics: ! 1. In a thermal cycle, heat energy cannot be completely transformed into mechanical work. ! 2. It is impossible to construct an operational perpetual-motion machine. ! 3. It’s impossible for any process to have as its sole result the transfer of heat from a cooler to a hotter body ! 4. Heat flows naturally from a hot object to a cold object; heat will not flow spontaneously from a cold object to a hot object. ! ! Heat Engines and Thermal Pumps A heat engine converts heat energy into work. According to the second law of thermodynamics, however, it cannot convert *all* of the heat energy supplied to it into work. Basic heat engine: hot reservoir, cold reservoir, and a machine to convert heat energy into work. Heat Engines and Thermal Pumps 4 Heat Engines and Thermal Pumps This is a simplified diagram of a heat engine, along with its thermal cycle. Heat Engines and Thermal Pumps An important quantity characterizing a heat engine is the net work it does when going through an entire cycle. Heat Engines and Thermal Pumps Heat Engines and Thermal Pumps Thermal efciency of a heat engine: ! ! ! ! ! ! From the first law, it follows: Heat Engines and Thermal Pumps Yet another restatement of the second law of thermodynamics: No cyclic heat engine can convert its heat input completely to work. Heat Engines and Thermal Pumps A thermal pump is the opposite of a heat engine: it transfers heat energy from a cold reservoir to a hot one. -

Physics 100 Lecture 7
2 Physics 100 Lecture 7 Heat Engines and the 2nd Law of Thermodynamics February 12, 2018 3 Thermal Convection Warm fluid is less dense and rises while cool fluid sinks Resulting circulation efficiently transports thermal energy 4 COLD Convection HOT Turbulent motion of glycerol in a container heated from below and cooled from above. The bright lines show regions of rapid temperature variation. The fluid contains many "plumes," especially near the walls. The plumes can be identified as mushroom-shaped objects with heat flowing through the "stalk" and spreading in the "cap." The hot plumes tend to rise with their caps on top; falling, cold plumes are cap-down. All this plume activity is carried along in an overall counterclockwise "wind" caused by convection. Note the thermometer coming down from the top of the cell. Figure adapted from J. Zhang, S. Childress, A. Libchaber, Phys. Fluids 9, 1034 (1997). See detailed discussion in Kadanoff, L. P., Physics Today 54, 34 (August 2001). 5 The temperature of land changes more quickly than the nearby ocean. Thus convective “sea breezes” blow ____ during the day and ____ during the night. A. onshore … onshore B. onshore … offshore C. offshore … onshore D. offshore … offshore 6 The temperature of land changes more quickly than the nearby ocean. Thus convective “sea breezes” blow ____ during the day and ____ during the night. A. onshore … onshore B.onshore … offshore C.offshore … onshore D.offshore … offshore 7 Thermal radiation Any object whose temperature is above zero Kelvin emits energy in the form of electromagnetic radiation Objects both absorb and emit EM radiation continuously, and this phenomenon helps determine the object’s equilibrium temperature 8 The electromagnetic spectrum 9 Thermal radiation We’ll examine this concept some more in chapter 6 10 Why does the Earth cool more quickly on clear nights than it does on cloudy nights? A. -

Fuel Cells Versus Heat Engines: a Perspective of Thermodynamic and Production
Fuel Cells Versus Heat Engines: A Perspective of Thermodynamic and Production Efficiencies Introduction: Fuel Cells are being developed as a powering method which may be able to provide clean and efficient energy conversion from chemicals to work. An analysis of their real efficiencies and productivity vis. a vis. combustion engines is made in this report. The most common mode of transportation currently used is gasoline or diesel engine powered automobiles. These engines are broadly described as internal combustion engines, in that they develop mechanical work by the burning of fossil fuel derivatives and harnessing the resultant energy by allowing the hot combustion product gases to expand against a cylinder. This arrangement allows for the fuel heat release and the expansion work to be performed in the same location. This is in contrast to external combustion engines, in which the fuel heat release is performed separately from the gas expansion that allows for mechanical work generation (an example of such an engine is steam power, where fuel is used to heat a boiler, and the steam then drives a piston). The internal combustion engine has proven to be an affordable and effective means of generating mechanical work from a fuel. However, because the majority of these engines are powered by a hydrocarbon fossil fuel, there has been recent concern both about the continued availability of fossil fuels and the environmental effects caused by the combustion of these fuels. There has been much recent publicity regarding an alternate means of generating work; the hydrogen fuel cell. These fuel cells produce electric potential work through the electrochemical reaction of hydrogen and oxygen, with the reaction product being water. -

Recording and Evaluating the Pv Diagram with CASSY
LD Heat Physics Thermodynamic cycle Leaflets P2.6.2.4 Hot-air engine: quantitative experiments The hot-air engine as a heat engine: Recording and evaluating the pV diagram with CASSY Objects of the experiment Recording the pV diagram for different heating voltages. Determining the mechanical work per revolution from the enclosed area. Principles The cycle of a heat engine is frequently represented as a closed curve in a pV diagram (p: pressure, V: volume). Here the mechanical work taken from the system is given by the en- closed area: W = − ͛ p ⋅ dV (I) The cycle of the hot-air engine is often described in an idealised form as a Stirling cycle (see Fig. 1), i.e., a succession of isochoric heating (a), isothermal expansion (b), isochoric cooling (c) and isothermal compression (d). This description, however, is a rough approximation because the working piston moves sinusoidally and therefore an isochoric change of state cannot be expected. In this experiment, the pV diagram is recorded with the computer-assisted data acquisition system CASSY for comparison with the real behaviour of the hot-air engine. A pressure sensor measures the pressure p in the cylinder and a displacement sensor measures the position s of the working piston, from which the volume V is calculated. The measured values are immediately displayed on the monitor in a pV diagram. Fig. 1 pV diagram of the Stirling cycle 0210-Wei 1 P2.6.2.4 LD Physics Leaflets Setup Apparatus The experimental setup is illustrated in Fig. 2. 1 hot-air engine . 388 182 1 U-core with yoke . -

Measuring the Steady State Thermal Efficiency of a Heating System
Measuring the Steady State Thermal Efficiency of a Heating System. Last week you had a tour of “Colby Power and Light” and had a chance to see Gus Libby’s really cool, campus-scale, multi-million dollar calorimeter. This week we will use lab-scale calorimeters to measure the heat and carbon content of different fuels (methane, propane, butane, and gasoline). Our simple calorimeters were designed by Whitney King and Chuck Jones, Science Division Instrument Wizard, to simulate the highly efficient HTP Versa Flame fire-tube boiler. The lab has three parts: 1) Calibrate the calorimeter 2) Measure the heat content of different fuels 3) Compare the measured heat content to the calculated heat content 1) Calibrate the Calorimeter. You lab instructor will demonstrate the assembly of the laboratory- scale fire-tube calorimeter. The heat transferred from the burning fuel to the calorimeter can be determined from the mass of the object being heated, the heat capacity of the object and the change in temperature. � = ���� ∗ �� ∗ ∆� We will begin the experiment by adding a known amount of heat to our calorimeters from a coffee cup heater. The heater is a simple resistor that converts electrical energy into heat. The heater has a resistance of 47.5 ohms and is plugged into a 120 V outlet. Recalling your electricity basics, we know that V=IR where V is the voltage in Figure 1. HTP Versa Flame Fire Tube volts, I is the current in amps, and R is the Boiler. Brown Tubes carry the hot resistance in ohms. The power, P, consumed by combustion gasses inside of the the heater is volts times current, water tank where they condense releasing the maximum available 2 heat. -

Thermodynamics of Power Generation
THERMAL MACHINES AND HEAT ENGINES Thermal machines ......................................................................................................................................... 1 The heat engine ......................................................................................................................................... 2 What it is ............................................................................................................................................... 2 What it is for ......................................................................................................................................... 2 Thermal aspects of heat engines ........................................................................................................... 3 Carnot cycle .............................................................................................................................................. 3 Gas power cycles ...................................................................................................................................... 4 Otto cycle .............................................................................................................................................. 5 Diesel cycle ........................................................................................................................................... 8 Brayton cycle ..................................................................................................................................... -

Power Plant Steam Cycle Theory - R.A
THERMAL POWER PLANTS – Vol. I - Power Plant Steam Cycle Theory - R.A. Chaplin POWER PLANT STEAM CYCLE THEORY R.A. Chaplin Department of Chemical Engineering, University of New Brunswick, Canada Keywords: Steam Turbines, Carnot Cycle, Rankine Cycle, Superheating, Reheating, Feedwater Heating. Contents 1. Cycle Efficiencies 1.1. Introduction 1.2. Carnot Cycle 1.3. Simple Rankine Cycles 1.4. Complex Rankine Cycles 2. Turbine Expansion Lines 2.1. T-s and h-s Diagrams 2.2. Turbine Efficiency 2.3. Turbine Configuration 2.4. Part Load Operation Glossary Bibliography Biographical Sketch Summary The Carnot cycle is an ideal thermodynamic cycle based on the laws of thermodynamics. It indicates the maximum efficiency of a heat engine when operating between given temperatures of heat acceptance and heat rejection. The Rankine cycle is also an ideal cycle operating between two temperature limits but it is based on the principle of receiving heat by evaporation and rejecting heat by condensation. The working fluid is water-steam. In steam driven thermal power plants this basic cycle is modified by incorporating superheating and reheating to improve the performance of the turbine. UNESCO – EOLSS The Rankine cycle with its modifications suggests the best efficiency that can be obtained from this two phaseSAMPLE thermodynamic cycle wh enCHAPTERS operating under given temperature limits but its efficiency is less than that of the Carnot cycle since some heat is added at a lower temperature. The efficiency of the Rankine cycle can be improved by regenerative feedwater heating where some steam is taken from the turbine during the expansion process and used to preheat the feedwater before it is evaporated in the boiler. -

25 Kw Low-Temperature Stirling Engine for Heat Recovery, Solar, and Biomass Applications
25 kW Low-Temperature Stirling Engine for Heat Recovery, Solar, and Biomass Applications Lee SMITHa, Brian NUELa, Samuel P WEAVERa,*, Stefan BERKOWERa, Samuel C WEAVERb, Bill GROSSc aCool Energy, Inc, 5541 Central Avenue, Boulder CO 80301 bProton Power, Inc, 487 Sam Rayburn Parkway, Lenoir City TN 37771 cIdealab, 130 W. Union St, Pasadena CA 91103 *Corresponding author: [email protected] Keywords: Stirling engine, waste heat recovery, concentrating solar power, biomass power generation, low-temperature power generation, distributed generation ABSTRACT This paper covers the design, performance optimization, build, and test of a 25 kW Stirling engine that has demonstrated > 60% of the Carnot limit for thermal to electrical conversion efficiency at test conditions of 329 °C hot side temperature and 19 °C rejection temperature. These results were enabled by an engine design and construction that has minimal pressure drop in the gas flow path, thermal conduction losses that are limited by design, and which employs a novel rotary drive mechanism. Features of this engine design include high-surface- area heat exchangers, nitrogen as the working fluid, a single-acting alpha configuration, and a design target for operation between 150 °C and 400 °C. 1 1. INTRODUCTION Since 2006, Cool Energy, Inc. (CEI) has designed, fabricated, and tested five generations of low-temperature (150 °C to 400 °C) Stirling engines that drive internally integrated electric alternators. The fifth generation of engine built by Cool Energy is rated at 25 kW of electrical power output, and is trade-named the ThermoHeart® Engine. Sources of low-to-medium temperature thermal energy, such as internal combustion engine exhaust, industrial waste heat, flared gas, and small-scale solar heat, have relatively few methods available for conversion into more valuable electrical energy, and the thermal energy is usually wasted. -
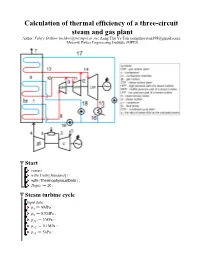
Calculation of Thermal Efficiency of a Three-Circuit Steam and Gas Plant
Calculation of thermal efficiency of a three-circuit steam and gas plant Author: Valery Ochkov ([email protected]), Aung Thu Ya Tun ([email protected]), Moscow Power Engineering Institute (MPEI) Start > > > > Steam turbine cycle Input data: > > > > > > > > > > > > > > Functions and procedures The specific enthalpy of the steam entering the turbine HPP: > (2.1) The specific enthalpy of steam at outlet of the turbine HPP: > (2.2) Specific work of HPP steam turbine: > (2.3) The temperature of the steam at the outlet of the HPP turbine: > (2.4) The specific enthalpy of steam at the point 10: > (2.5) > (2.6) The balance of the mixing streams at points 9 and 10: > (2.7) The temperature of steam at the inlet to the MPP of the turbine: > (2.8) > (2.8) > (2.9) > Specific enthalpy of water vapor at the inlet to the MPP of the turbine: > (2.10) Specific enthalpy of steam at the outlet of the turbine MPP: > (2.11) C : > (2.12) The temperature of the steam at the outlet of the MPP of the turbine: > (2.13) The specific enthalpy of steam at point 13: > (2.14) > (2.15) Balance when mixing streams at points 12 and 13: > (2.16) The temperature of steam at the inlet to the LPP of the turbine: > (2.17) > (2.18) The specific enthalpy of steam at outlet of the turbine LPP: > (2.19) Specific LPP operation of a steam turbine: > (2.8) > (2.20) The degree of dryness of the steam at the outlet of the turbine LPP: > (2.21) Condensate temperature: > (2.22) Specific enthalpy of condensate: > (2.23) The specific enthalpy of the feed water high pressure -

Numerical Analysis of the 4 K Regenerator in a Pulse Tube Cryocooler
263 Numerical Analysis of the 4 K Regenerator in a Pulse Tube Cryocooler Y.Zhao1,2, W.Dai1, Y.Chen1, X.Wang1, E.Luo1 1Key Laboratory of Cryogenics, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190 China 2University of Chinese Academy of Sciences, Beijing 100049, China ABSTRACT The numerical simulation of a 4 K pulse tube cryocooler is difficult due to its complexity of nature. The main difficulty comes from the oscillatory flow combined with the real gas effects. In this paper, the numerical simulation of the 4 K regenerator in a pulse tube cryocooler with a relatively high frequency has been performed with the commercial CFD software, FLUENT [1]. FLUENT takes into account the non-ideal gas properties of 4He, the properties of the magnetic regenerative material, and the heat transfer inside the heat exchanger and regenerator. Interesting features such as the acoustic work flow, temperature distribution, the volume flow, etc., have been systematically studied. A cooling power of 0.36 W was obtained numerically at the temperature of 4.2 K. The study takes a deeper look into the working mechanism of a 4 K pulse tube cryocooler as well as setting the basis for optimizing a pulse tube cooler driven by a Vuillemier type thermal compressor. INTRODUCTION Cryocoolers working at liquid helium temperatures could be used in the application fields of space exploration, military, medical and low-temperature physics. Especially, for many applications, a 4 K pulse tube cryocooler is a potential candidate because of the merits of no cryogenic moving components. Owing to the advances in cooler design and the development of magnetic regenerative materials [2-4], a cooling temperature below 4 K was first obtained with a three-stage Gifford McMahon (G-M) type pulse tube refrigerator [5]. -

Stirling Engine)
Heat Cycles Hot air engine (Stirling engine) OPERATE A FUNCTIONAL MODEL OF A STIRLING ENGINE AS A HEAT ENGINE Operate the hot-air engine as a heat engine Demonstrate how thermal energy is converted into mechanical energy Measure the no-load speed as a function of the thermal power UE2060100 04/16 JS BASIC PRINCIPLES The thermodynamic cycle of the Stirling engine While the working piston is in its top dead centre posi- (invented by Rev. R. Stirling in 1816) can be pre- tion: the displacement piston retracts and air is dis- sented in a simplified manner as the processes placed towards the top end of the large cylinder so that thermal input, expansion, thermal output and com- it cools. pression. These processes have been illustrated by The cooled air is compressed by the working piston schematic diagrams (Fig. 1 to Fig. 4) for the func- extending. The mechanical work required for this is tional model used in the experiment. provided by the flywheel rod. A displacement piston P1 moves upwards and displac- If the Stirling engine is operated without any mechanical es the air downwards into the heated area of the large load, it operates with at a speed which is limited only by cylinder, thereby facilitating the input of air. During this internal friction and which depends on the input heating operation the working piston is at its bottom dead centre energy. The speed is reduced as soon as a load takes position since the displacement piston is ahead of the up some of the mechanical energy. This is most easily working piston by 90°. -

Methodology for Thermal Efficiency and Energy Input Calculations and Analysis of Biomass Cogeneration Unit Characteristics
Technical Support Document for the Revisions to Definition of Cogeneration Unit in Clean Air Interstate Rule (CAIR), CAIR Federal Implementation Plan, Clean Air Mercury Rule (CAMR), and CAMR Proposed Federal Plan; Revision to National Emission Standards for Hazardous Air Pollutants for Industrial, Commercial, and Institutional Boilers and Process Heaters; and Technical Corrections to CAIR and Acid Rain Program Rules Methodology for Thermal Efficiency and Energy Input Calculations and Analysis of Biomass Cogeneration Unit Characteristics EPA Docket number: EPA-HQ-OAR-2007-0012 April 2007 U.S. Environmental Protection Agency Office of Air and Radiation Technical Support Document for Proposed Revisions to Cogeneration Definition in CAIR, CAIR FIP, CAMR, and Proposed CAMR Federal Plan This Technical Support Document (TSD) has several purposes. One purpose of the TSD is to set forth the methodology for determining the thermal efficiency of a unit for purposes of applying the definition of the term “cogeneration unit” under the existing CAIR, the CAIR model trading rules, the CAIR FIP, CAMR, the CAMR Hg model trading rule, and the proposed CAMR Federal Plan. Another purpose of the TSD is to present information relevant to the proposed revisions, and other potential revisions for which EPA is requesting comment, concerning the thermal efficiency standard. One of the critical values used in the determination of thermal efficiency is the “total energy input” of the unit. Consequently, in connection with setting forth the methodology for determining thermal efficiency, the TSD specifically addresses what formula is to be used in calculating a unit’s total energy input under the existing rules. There are two major issues concerning the calculation of total energy input.