CALCAREOUS ALGAE Bibliography CALCIFICATION CALCIFIED
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sediment Activity Answer Key
Sediment Activity Answer Key 1. Were your predictions close to where calcareous and siliceous oozes actually occur? Answers vary. 2. How does your map compare with the sediment distribution map? Answers vary. 3. Which type of ooze dominates the ocean sediments, calcareous or siliceous? Why? Calcareous sediments are formed from the remains of organisms like plankton with calcium-based skeletons1, such as foraminifera, while siliceous ooze is formed from the remains of organisms with silica-based skeletons like diatoms or radiolarians. Calcareous ooze dominates ocean sediments. Organisms with calcium-based shells such as foraminifera are abundant and widely distributed throughout the world’s ocean basins –more so than silica-based organisms. Silica-based phytoplankton such as diatoms are more limited in distribution by their (higher) nutrient requirements and temperature ranges. 4. What parts of the ocean do not have calcareous ooze? What might be some reasons for this? Remember that ooze forms when remains of organisms compose more than 30% of the sediment. The edges of ocean basins bordering land tend to have a greater abundance of lithogenous sediment –sediment that is brought into the ocean by water and wind. The proportion of lithogenous sediment decreases however as you move away from the continental shelf. In nutrient rich areas such as upwelling zones in the polar and equatorial regions, silica-based organisms such as diatoms or radiolarians will dominate, making the sediments more likely to be a siliceous-based ooze. Further, factors such as depth, temperature, and pressure can affect the ability of calcium carbonate to dissolve. Areas of the ocean that lie beneath the carbonate compensation depth (CCD), below which calcium carbonate dissolves, typically beneath 4-5 km, will be dominated by siliceous ooze because calcium-carbonate-based material would dissolve in these regions. -

Sarcoidosis, Hypercalcemia and Calcium-Sensing Receptor Mutation: a Case Report
468 Letters to the Editor Sarcoidosis, Hypercalcemia and Calcium-sensing Receptor Mutation: A Case Report Shreya Dixit1 and Pablo Fernandez-Peñas1,2* 1Skin and Cancer Foundation Australia, 7 Ashley Lane, Westmead, NSW 2145, and 2Sydney Medical School Western, The University of Sydney, NSW, Australia. *E-mail: [email protected] Accepted October 28, 2010. Sarcoidosis is a multisystem granulomatous disease of The incidence of hypercalcaemia in patients with unknown aetiology. It predominantly affects the lungs; sarcoidosis is approximately 10% (3). It has previously however, 25% of patients have skin involvement, ranging been found that these abnormalities of calcium metabo- from non-specific maculopapular eruptions to erythema lism are due to dysregulated production of calcitriol by nodosum and lupus pernio (1). activated macrophages trapped in pulmonary alveoli and Familial hypocalciuric hypercalcaemia (FHH) results granulomatous inflammation (4). Conversion of calcidiol from an autosomal dominantly inherited inactivating to calcitriol is facilitated by 1 alpha-hydroxylase, a cyto- mutation of the calcium-sensing receptor (CaSR) gene chrome P450 enzyme. The activity of 1 alpha-hydroxyla- and is typically asymptomatic (2). Sarcoidosis has not se is tightly regulated through complex mechanisms that previously been reported in FHH. We had the opportu- depend on the circulating levels of calcium, phosphorus, nity to study a patient with sarcoidosis that has a CaSR PTH, 1,25 (OH)2D3 (calcitriol) and calcitonin. The role mutation. of CaSR in the regulation of 1 alpha-hydroxylase has not been defined, although there are suggestions that CaSR activation can repress the enzyme (5). CASE REPORT The incidence of FHH in patients with sarcoidosis A 70-year-old woman was referred by her endocrinologist with is unknown. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -

Calcareous Soils Are Alkaline
By Mongi Zekri, Tom Obreza and Kelly Morgan alcareous soils are alkaline (pH > 7) due to the pres- ence of excess calcium carbonate (CaCO3). These soils Ccan contain from 1 percent to more than 25 percent CaCO3 by weight, with pH in the range of 7.6 to 8.4. In Florida, soil pH is usually not higher than 8.4 regardless of CaCO3 concentration. Many Florida flatwoods soils contain one or more hori- zons (layers) that are calcareous. A typical characteristic is an alkaline, loamy horizon less than 40 inches deep that can be brought to the surface during land preparation for citrus Calcareous soil in Southwest Florida planting. Increased nutritional management intensity is re- quired to successfully grow citrus on calcareous soils. Some lution of fixed P. Applied P is available to replenish the soil grove soils (e.g. ditch banks) contain considerable amounts solution for only a relatively short time before it converts to of lime rock or shell. It may not be economically justifiable less soluble forms of P. To maintain P availability to citrus to plant these sites with certain rootstocks considering the on calcareous soils, water-soluble P fertilizer should be ap- management problems and costs involved. plied on a regular, but not necessarily frequent, basis. Since Citrus fertilizer management on calcareous soils differs P accumulates in the soil, it is at least partially available as from that on non-calcareous soils because the presence of it converts to less soluble compounds with time. CaCO3 directly or indirectly affects plant availability of N, Potassium (K) P, K, Calcium (Ca), Mg, Mn, Zn, Fe and Cu. -
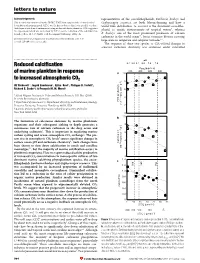
Reduced Calcification of Marine Plankton in Response to Increased
letters to nature Acknowledgements representatives of the coccolithophorids, Emiliania huxleyi and This research was sponsored by the EPSRC. T.W.F. ®rst suggested the electrochemical Gephyrocapsa oceanica, are both bloom-forming and have a deoxidation of titanium metal. G.Z.C. was the ®rst to observe that it was possible to reduce world-wide distribution. G. oceanica is the dominant coccolitho- thick layers of oxide on titanium metal using molten salt electrochemistry. D.J.F. suggested phorid in neritic environments of tropical waters9, whereas the experiment, which was carried out by G.Z.C., on the reduction of the solid titanium dioxide pellets. M. S. P. Shaffer took the original SEM image of Fig. 4a. E. huxleyi, one of the most prominent producers of calcium carbonate in the world ocean10, forms extensive blooms covering Correspondence and requests for materials should be addressed to D. J. F. large areas in temperate and subpolar latitudes9,11. (e-mail: [email protected]). The response of these two species to CO2-related changes in seawater carbonate chemistry was examined under controlled ................................................................. pH Reduced calci®cation 8.4 8.2 8.1 8.0 7.9 7.8 PCO2 (p.p.m.v.) of marine plankton in response 200 400 600 800 a 10 to increased atmospheric CO2 ) 8 –1 Ulf Riebesell *, Ingrid Zondervan*, BjoÈrn Rost*, Philippe D. Tortell², d –1 Richard E. Zeebe*³ & FrancËois M. M. Morel² 6 * Alfred Wegener Institute for Polar and Marine Research, P.O. Box 120161, 4 D-27515 Bremerhaven, Germany mol C cell –13 ² Department of Geosciences & Department of Ecology and Evolutionary Biology, POC production Princeton University, Princeton, New Jersey 08544, USA (10 2 ³ Lamont-Doherty Earth Observatory, Columbia University, Palisades, New York 10964, USA 0 ............................................................................................................................................. -

Occurrence of Nitrogen-Fixing Cyanobacteria During Different Stages of Paddy Cultivation
Bangladesh J. Plant Taxon. 18(1): 73-76, 2011 (June) ` - Short communication © 2011 Bangladesh Association of Plant Taxonomists OCCURRENCE OF NITROGEN-FIXING CYANOBACTERIA DURING DIFFERENT STAGES OF PADDY CULTIVATION * KAUSHAL KISHORE CHOUDHARY Department of Botany, B.R.A. Bihar University, Muzaffarpur-842001, Bihar, India Keywords: Cyanobacteria; Diversity; Nitrogen-fixing; Rice fields; North Bihar. Rapid decline in soil fertility and productivity due to excessive application of chemical fertilizer particularly nitrogen and its increasing cost has induced to develop alternate biological sources of nitrogenous fertilizers (Boussiba, 1991). Biological fertilizers maintain the nitrogen status of the soils and helps in optimum crop production to meet the demand of increasing human populations while maintaining the agricultural practices sustainable. With establishment of agronomic potential of cyanobacteria (Singh, 1950), these photosynthetic prokaryotes were applied and studied for enrichment of different living ecosystems with nitrogenous compounds. Cyanobacteria are endowed with a specialized structure ‘heterocyst’ with ‘nitrogenase complex’ capable of converting unavailable sources of molecular nitrogen into nitrogenous compounds (Ernst et al., 1992). The ability of cyanobacteria to fix atmospheric nitrogen is increasing concern worldwide to exploit this tiny living system for nitrogenous fertilizers for sustainable agriculture practices. Advances in cyanobacteria have revealed their significant contribution in promoting the fertility of the soil and water including marine by adding nitrogen and phosphorus. Cyanobacteria contribute phosphorus to the soil by mobilizing the insoluble organic phosphates present in the soil with enzyme ‘phosphatses’ (Whitton et al., 1991). Moreover, cyanobacteria enhance the water holding capacity by adding polysaccharidic material to the soil (Richert et al., 2005) that increases the soil aggregation property. -

Distribution of Calcium Phosphate in the Exoskeleton of Larval Exeretonevra Angustifrons Hardy (Diptera: Xylophagidae)
Arthropod Structure & Development 34 (2005) 41–48 www.elsevier.com/locate/asd Distribution of calcium phosphate in the exoskeleton of larval Exeretonevra angustifrons Hardy (Diptera: Xylophagidae) Bronwen W. Cribba,b,*, Ron Rascha, John Barrya, Christopher M. Palmerb,1 aCentre for Microscopy and Microanalysis, The University of Queensland, Brisbane, Qd 4072, Australia bDepartment of Zoology and Entomology, The University of Queensland, Brisbane, Qd 4072, Australia Received 28 July 2004; accepted 26 August 2004 Abstract Distribution and organisation of the mineral, amorphous calcium phosphate (ACP), has been investigated in the exoskeleton of the xylophagid fly larva Exeretonevra angustifrons Hardy. While head capsule and anal plate are smooth with a thin epicuticle, the epicuticle of the body is thicker and shows unusual micro-architecture comprised of minute hemispherical (dome-shaped) protrusions. Electron microprobe analysis and energy dispersive spectroscopy revealed heterogeneity of mineral elements across body cuticle and a concentration of ACP in the epicuticle, especially associated with the hemispherical structures. Further imaging and analysis showed the bulk of the ACP to be present in nano-sized granules. It is hypothesised that the specific distribution of ACP may enhance cuticular hardness or durability without reducing flexibility. q 2004 Elsevier Ltd. All rights reserved. Keywords: Insect; Cuticle; Integument; Hardening; Analytical electron microscopy; Electron microprobe 1. Introduction was distributed heterogeneously. Further investigation of the distribution of the mineral phase at the micron and Strengthening of biological structures through cuticular nanometre level is needed to discover where deposition is calcification is well developed in decapod crustaceans but it occurring and how this might affect exoskeletal organis- rarely occurs in insects, where it is poorly understood ation. -

Part 629 – Glossary of Landform and Geologic Terms
Title 430 – National Soil Survey Handbook Part 629 – Glossary of Landform and Geologic Terms Subpart A – General Information 629.0 Definition and Purpose This glossary provides the NCSS soil survey program, soil scientists, and natural resource specialists with landform, geologic, and related terms and their definitions to— (1) Improve soil landscape description with a standard, single source landform and geologic glossary. (2) Enhance geomorphic content and clarity of soil map unit descriptions by use of accurate, defined terms. (3) Establish consistent geomorphic term usage in soil science and the National Cooperative Soil Survey (NCSS). (4) Provide standard geomorphic definitions for databases and soil survey technical publications. (5) Train soil scientists and related professionals in soils as landscape and geomorphic entities. 629.1 Responsibilities This glossary serves as the official NCSS reference for landform, geologic, and related terms. The staff of the National Soil Survey Center, located in Lincoln, NE, is responsible for maintaining and updating this glossary. Soil Science Division staff and NCSS participants are encouraged to propose additions and changes to the glossary for use in pedon descriptions, soil map unit descriptions, and soil survey publications. The Glossary of Geology (GG, 2005) serves as a major source for many glossary terms. The American Geologic Institute (AGI) granted the USDA Natural Resources Conservation Service (formerly the Soil Conservation Service) permission (in letters dated September 11, 1985, and September 22, 1993) to use existing definitions. Sources of, and modifications to, original definitions are explained immediately below. 629.2 Definitions A. Reference Codes Sources from which definitions were taken, whole or in part, are identified by a code (e.g., GG) following each definition. -

Mid-Depth Calcareous Contourites in the Latest Cretaceous of Caravaca (Subbetic Zone, SE Spain)
Mid-depth calcareous contourites in the latest Cretaceous of Caravaca (Subbetic Zone, SE Spain). Origin and palaeohydrological significance Javier Martin-Chivelet*, Maria Antonia Fregenal-Martinez, Beatriz Chac6n Departamento de 8stratigrajia. institute de Geologia Economica (CSiC-UCM). Facultad de Ciencias Geologicas. Universidad Complutense. 28040 Madrid, Spain Abstract Deep marine carbonates of Late Campanian to Early Maastrichtian age that crop out in the Subbetic Zone near Caravaca (SE Spain) contain a thick succession of dm-scale levels of calcareous contourites, alternating with fine-grained pelagitesl hemipelagites. These contourites, characterised by an abundance and variety of traction structures, internal erosive surfaces and inverse and nOlmal grading at various scales, were interpreted as having been deposited under the influence of relatively deep ocean CUlTents. Based on these contourites, a new facies model is proposed. The subsurface currents that generated the contourites of Caravaca were probably related to the broad circumglobal, equatorial current system, the strongest oceanic feature of Cretaceous times. These deposits were formed in the mid-depth (200-600 m), hemipelagic environments at the ancient southern margin of Iberia. This palaeogeographic setting was susceptible to the effects of these currents because of its position close to the narrowest oceanic passage, through which the broad equatorial cun'ent system flowed in the westemmost area of the Tethys Seaway. Regional uplift, related to the onset of convergence between Iberia and Africa, probably favoured the generation of the contourites during the Late Campanian to the Early Maastrichtian. Keyword\': Contourites; Palaeoceanography; Late Cretaceous; Caravaca; Betics; SE Spain 1. Introduction aI., 1996; Stow and Faugeres, 1993, 1998; Stow and Mayall, 2000a; Shanmugam, 2000). -

Algal Toxic Compounds and Their Aeroterrestrial, Airborne and Other Extremophilic Producers with Attention to Soil and Plant Contamination: a Review
toxins Review Algal Toxic Compounds and Their Aeroterrestrial, Airborne and other Extremophilic Producers with Attention to Soil and Plant Contamination: A Review Georg G¨аrtner 1, Maya Stoyneva-G¨аrtner 2 and Blagoy Uzunov 2,* 1 Institut für Botanik der Universität Innsbruck, Sternwartestrasse 15, 6020 Innsbruck, Austria; [email protected] 2 Department of Botany, Faculty of Biology, Sofia University “St. Kliment Ohridski”, 8 blvd. Dragan Tsankov, 1164 Sofia, Bulgaria; mstoyneva@uni-sofia.bg * Correspondence: buzunov@uni-sofia.bg Abstract: The review summarizes the available knowledge on toxins and their producers from rather disparate algal assemblages of aeroterrestrial, airborne and other versatile extreme environments (hot springs, deserts, ice, snow, caves, etc.) and on phycotoxins as contaminants of emergent concern in soil and plants. There is a growing body of evidence that algal toxins and their producers occur in all general types of extreme habitats, and cyanobacteria/cyanoprokaryotes dominate in most of them. Altogether, 55 toxigenic algal genera (47 cyanoprokaryotes) were enlisted, and our analysis showed that besides the “standard” toxins, routinely known from different waterbodies (microcystins, nodularins, anatoxins, saxitoxins, cylindrospermopsins, BMAA, etc.), they can produce some specific toxic compounds. Whether the toxic biomolecules are related with the harsh conditions on which algae have to thrive and what is their functional role may be answered by future studies. Therefore, we outline the gaps in knowledge and provide ideas for further research, considering, from one side, Citation: G¨аrtner, G.; the health risk from phycotoxins on the background of the global warming and eutrophication and, ¨а Stoyneva-G rtner, M.; Uzunov, B. -

Limestone Resources of Western Washington
State of Washington DANIEL J. EVANS, Governor Department of Conservation H. MA URI CE AHLQUIST, Director DIVISION OF MINES AND GEOLOGY MARSHALL T. HUNTTING, Supervisor Bulletin No. 52 LIMESTONE RESOURCES OF WESTERN WASHINGTON By WILBERT R. DANNER With a section on the UME MOUNTAIN DEPOSIT By GERALD W. THORSEN STATII PRINTING PLANT, OLYMPI A, WASH, 1966 For sale by Department of Conservation, Olympia, Washington. Price, $4,50 FOREWORD Since the early days of Washington's statehood, limestone has been recognized as one of the important mineral resources _of the State. The second annual report of the Washington Geological Survey, published in 1903, gave details on the State's limestone deposits, and in later years five other reports published by the Survey and its successor agencies hove given additional information on this resource. Still other reports by Federal and private agencies hove been published in response to demands for data on limestone here. Although some of the earlier reports included analyses to show the purity of the rocks, very few of the samples for analysis were taken systemati cally in a way that would fairly represent the deposits sampled. Prior to 1900 limestone was produced for use as building stone here, and another important use was for the production of burned Ii me . Portland cement plants soon became leading consumers of Ii mestone, and they con tinue as such to the present time . Limestone is used in large quantities in the pulp industry in the Northwest, and in 1966 there was one commercial lime-burning plant in the State. Recognizing the potential for industrial development in Washington based on more intensive use of our mineral resources, and recognizing the need to up-dote the State's knowledge of raw material resources in order to channel those resources into the State's growing economy, the Industrial Row Materials Advisory Committee of the Deportment of Commerce and Economic Development in 1958 recommended that a comprehensive survey be made of the limestone resources of Washington. -

DOMAIN Bacteria PHYLUM Cyanobacteria
DOMAIN Bacteria PHYLUM Cyanobacteria D Bacteria Cyanobacteria P C Chroobacteria Hormogoneae Cyanobacteria O Chroococcales Oscillatoriales Nostocales Stigonematales Sub I Sub III Sub IV F Homoeotrichaceae Chamaesiphonaceae Ammatoideaceae Microchaetaceae Borzinemataceae Family I Family I Family I Chroococcaceae Borziaceae Nostocaceae Capsosiraceae Dermocarpellaceae Gomontiellaceae Rivulariaceae Chlorogloeopsaceae Entophysalidaceae Oscillatoriaceae Scytonemataceae Fischerellaceae Gloeobacteraceae Phormidiaceae Loriellaceae Hydrococcaceae Pseudanabaenaceae Mastigocladaceae Hyellaceae Schizotrichaceae Nostochopsaceae Merismopediaceae Stigonemataceae Microsystaceae Synechococcaceae Xenococcaceae S-F Homoeotrichoideae Note: Families shown in green color above have breakout charts G Cyanocomperia Dactylococcopsis Prochlorothrix Cyanospira Prochlorococcus Prochloron S Amphithrix Cyanocomperia africana Desmonema Ercegovicia Halomicronema Halospirulina Leptobasis Lichen Palaeopleurocapsa Phormidiochaete Physactis Key to Vertical Axis Planktotricoides D=Domain; P=Phylum; C=Class; O=Order; F=Family Polychlamydum S-F=Sub-Family; G=Genus; S=Species; S-S=Sub-Species Pulvinaria Schmidlea Sphaerocavum Taxa are from the Taxonomicon, using Systema Natura 2000 . Triochocoleus http://www.taxonomy.nl/Taxonomicon/TaxonTree.aspx?id=71022 S-S Desmonema wrangelii Palaeopleurocapsa wopfnerii Pulvinaria suecica Key Genera D Bacteria Cyanobacteria P C Chroobacteria Hormogoneae Cyanobacteria O Chroococcales Oscillatoriales Nostocales Stigonematales Sub I Sub III Sub