Limestone Resources of Western Washington
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Source and Bedrock Distribution of Gold and Platinum-Group Metals in the Slate Creek Area, Northern.Chistochina Mining District, East-Central Alaska
Source and Bedrock Distribution of Gold and Platinum-Group Metals in the Slate Creek Area, Northern.Chistochina Mining District, East-Central Alaska By: Jeffrey Y. Foley and Cathy A. Summers Open-file report 14-90******************************************1990 UNITED STATES DEPARTMENT OF THE INTERIOR Manuel Lujan, Jr., Secretary BUREAU OF MINES T S Arv. Director TN 23 .U44 90-14 c.3 UNITED STATES BUREAU OF MINES -~ ~ . 4,~~~~1 JAMES BOYD MEMORIAL LIBRARY CONTENTS Abstract 1 Introduction 2 Acknowledgments 2 Location, access, and land status 2 History and production 4 Previous work 8 Geology 8 Regional and structural geologic setting 8 Rock units 8 Dacite stocks, dikes, and sills 8 Limestone 9 Argillite and sandstone 9 Differentiated igneous rocks north of the Slate Creek Fault Zone 10 Granitic rocks 16 Tertiary conglomerate 16 Geochemistry and metallurgy 18 Mineralogy 36 Discussion 44 Recommendations 45 References 47 ILLUSTRATIONS 1. Map of Slate Creek and surrounding area, in the northern Chistochina Mining District 3 2. Geologic map of the Slate Creek area, showing sample localities and cross section (in pocket) 3. North-dipping slaty argillite with lighter-colored sandstone intervals in lower Miller Gulch 10 4. North-dipping differentiated mafic and ultramafic sill capping ridge and overlying slaty argillite at upper Slate Creek 11 5. Dike swarm cutting Jurassic-Cretaceous turbidites in Miller Gulch 12 6 60-ft-wide diorite porphyry and syenodiorite porphyry dike at Miller Gulch 13 7. Map showing the locations of PGM-bearing mafic and ultramafic rocks and major faults in the east-central Alaska Range 14 8. Major oxides versus Thornton-Tuttle differentiation index 17 9. -
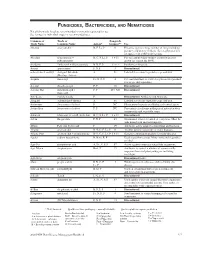
Fungicides, Bactericides, and Nematicides Not All Chemicals Listed Are Recommended Or Currently Registered for Use
FUNGICIDES, BACTERICIDES, AND NEMATICIDES Not all chemicals listed are recommended or currently registered for use. See listings for individual crops for recommended uses. Common or Trade or Fungicide Trade Name Common Name Action* Group #** Use Abound azoxystrobin B, F, Ls, P 11 Effective against a large number of fungi including powdery and downy mildews. Severe phytotoxicity on apples with a McIntosh heritage. Absolute tebuconazole + B, C, F, Ls, P 3 + 11 For rust and powdery mildew control in grasses trifloxystrobin grown for seed in the PNW. Academy fludioxonil + difenoconazole B-N, F, P 12 + 3 Postharvest fungicide. Accrue spiroxamine F, N, P 5 Discontinued. acibenzolar-S-methyl Actigard, Blockade A P1 Labeled for certain vegetable crops and fruit. (Heritage Action) Acquire metalaxyl Fs, N, P, S 4 For seed treatment to control ooymcetes in specified row crops and vegetables. Acrobat dimethomorph F, P 40 Discontinued. Acrobat MZ dimethomorph + F, P 40 + M3 Discontinued. mancozeb Acti-dione cycloheximide F Discontinued. Antibiotic and fungicide. Actigard acibenzolar-S-methyl A P1 Labeled for certain vegetable crops and fruit. Actinovate Streptomyces lydicus F NC Filamentous bacteria as a Biological control agent. Actino-Iron Streptomyces lydicus F, P NC For control of soilborne pathogens of indoor/outdoor ornamentals and vegetable crops. Adament tebuconazole + trifloxystrobin B, C, F, Ls, P 3 + 11 Discontinued. Adorn fluopicolide F, N, P 43 Ornamental label for control of oomycetes. Must be tank-mixed with another fungicide. Affirm Polyoxin D zinc salt F 19 Antibiotic active against certain fungi and bacteria. Aframe azoxystrobin B-N, C, F, Ls, P 11 Another generic fungicide for many diseases. -

Geology of the Nimrod Area Granite County Montana
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 1958 Geology of the Nimrod area Granite County Montana Joel Kenneth Montgomery The University of Montana Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Montgomery, Joel Kenneth, "Geology of the Nimrod area Granite County Montana" (1958). Graduate Student Theses, Dissertations, & Professional Papers. 7095. https://scholarworks.umt.edu/etd/7095 This Thesis is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. aUv DATE DUE .**'“ '■’ K ^-r>o<> . t_A \J ^ D e c 0 1 ]ggg ^ A H 1 5 GEOLOGY OP THE NIMROD AREA GRANITE COUNTY, MONTANA by JOEL K. MONTGOMERY B. S. Brigham Young University, 1956 Presented in partial fulfillment of the requirements for the degree Master of Science MONTANA STATE UNIVERSITY 1958 Approved by: Chairman, Board of ^aminers Dean, Graduate School 2 0 1953 bate UMl Number: EP37896 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. UMT OisMftartion Publishing UMl EP37896 Published by ProQuest LLC (2013). -

Sediment Activity Answer Key
Sediment Activity Answer Key 1. Were your predictions close to where calcareous and siliceous oozes actually occur? Answers vary. 2. How does your map compare with the sediment distribution map? Answers vary. 3. Which type of ooze dominates the ocean sediments, calcareous or siliceous? Why? Calcareous sediments are formed from the remains of organisms like plankton with calcium-based skeletons1, such as foraminifera, while siliceous ooze is formed from the remains of organisms with silica-based skeletons like diatoms or radiolarians. Calcareous ooze dominates ocean sediments. Organisms with calcium-based shells such as foraminifera are abundant and widely distributed throughout the world’s ocean basins –more so than silica-based organisms. Silica-based phytoplankton such as diatoms are more limited in distribution by their (higher) nutrient requirements and temperature ranges. 4. What parts of the ocean do not have calcareous ooze? What might be some reasons for this? Remember that ooze forms when remains of organisms compose more than 30% of the sediment. The edges of ocean basins bordering land tend to have a greater abundance of lithogenous sediment –sediment that is brought into the ocean by water and wind. The proportion of lithogenous sediment decreases however as you move away from the continental shelf. In nutrient rich areas such as upwelling zones in the polar and equatorial regions, silica-based organisms such as diatoms or radiolarians will dominate, making the sediments more likely to be a siliceous-based ooze. Further, factors such as depth, temperature, and pressure can affect the ability of calcium carbonate to dissolve. Areas of the ocean that lie beneath the carbonate compensation depth (CCD), below which calcium carbonate dissolves, typically beneath 4-5 km, will be dominated by siliceous ooze because calcium-carbonate-based material would dissolve in these regions. -

Mineralogy and Geochemistry of Some Belt Rocks, Montana and Idaho
Mineralogy and Geochemistry of Some Belt Rocks, Montana and Idaho By J. E. HARRISON and D. J. GRIMES CONTRIBUTIONS TO ECONOMIC GEOLOGY GEOLOGICAL SURVEY BULLETIN 1312-O A comparison of rocks from two widely separated areas in Belt terrane UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1970 UNITED STATES DEPARTMENT OF THE INTERIOR WALTER J. HICKEL, Secretary GEOLOGICAL SURVEY William T. Pecora, Director Library of Congress catalog-card No. 75-607766 4- V For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 Price 35 cents CONTENTS Page Abstract .................................................................................................................. Ol Introduction ............................:.................................... .......................................... 2 General geology ...............................................................;.'................................... 3 Methods of investigation ........................y .............................................................. 7 Sampling procedure ..............................:....................................................... 7 Analytical technique for mineralogy ...............:............................................ 11 Analytical technique and calculations for geochemistry................................ 13 Mineralogy of rock types........................................................................................ 29 Geochemistry ......................................................................................................... -

Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries
Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries. Peter Jentsch Extension Associate Department of Entomology Cornell University's Hudson Valley Lab 3357 Rt. 9W; PO box 727 Highland, NY 12528 email: [email protected] Phone 845-691-7151 Mobile: 845-417-7465 http://www.nysaes.cornell.edu/ent/faculty/jentsch/ 2 Historical Perspectives on Fruit Production: Fruit Tree Pest Management, Regulation and New Chemistries. by Peter Jentsch I. Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 Synthetic Pesticide Development and Use II. Influences Changing the Pest Management Profile in Apple Production Chemical Residues in Early Insect Management Historical Chemical Regulation Recent Regulation Developments Changing Pest Management Food Quality Protection Act of 1996 The Science Behind The Methodology Pesticide Revisions – Requirements For New Registrations III. Resistance of Insect Pests to Insecticides Resistance Pest Management Strategies IV. Reduced Risk Chemistries: New Modes of Action and the Insecticide Treadmill Fermentation Microbial Products Bt’s, Abamectins, Spinosads Juvenile Hormone Analogs Formamidines, Juvenile Hormone Analogs And Mimics Insect Growth Regulators Azadirachtin, Thiadiazine Neonicotinyls Major Reduced Risk Materials: Carboxamides, Carboxylic Acid Esters, Granulosis Viruses, Diphenyloxazolines, Insecticidal Soaps, Benzoyl Urea Growth Regulators, Tetronic Acids, Oxadiazenes , Particle Films, Phenoxypyrazoles, Pyridazinones, Spinosads, Tetrazines , Organotins, Quinolines. 3 I Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 The apple has a rather ominous origin. Its inception is framed in the biblical text regarding the genesis of mankind. The backdrop appears to be the turbulent setting of what many scholars believe to be present day Iraq. -

Bedrock Geology Glossary from the Roadside Geology of Minnesota, Richard W
Minnesota Bedrock Geology Glossary From the Roadside Geology of Minnesota, Richard W. Ojakangas Sedimentary Rock Types in Minnesota Rocks that formed from the consolidation of loose sediment Conglomerate: A coarse-grained sedimentary rock composed of pebbles, cobbles, or boul- ders set in a fine-grained matrix of silt and sand. Dolostone: A sedimentary rock composed of the mineral dolomite, a calcium magnesium car- bonate. Graywacke: A sedimentary rock made primarily of mud and sand, often deposited by turbidi- ty currents. Iron-formation: A thinly bedded sedimentary rock containing more than 15 percent iron. Limestone: A sedimentary rock composed of calcium carbonate. Mudstone: A sedimentary rock composed of mud. Sandstone: A sedimentary rock made primarily of sand. Shale: A deposit of clay, silt, or mud solidified into more or less a solid rock. Siltstone: A sedimentary rock made primarily of sand. Igneous and Volcanic Rock Types in Minnesota Rocks that solidified from cooling of molten magma Basalt: A black or dark grey volcanic rock that consists mainly of microscopic crystals of pla- gioclase feldspar, pyroxene, and perhaps olivine. Diorite: A plutonic igneous rock intermediate in composition between granite and gabbro. Gabbro: A dark igneous rock consisting mainly of plagioclase and pyroxene in crystals large enough to see with a simple magnifier. Gabbro has the same composition as basalt but contains much larger mineral grains because it cooled at depth over a longer period of time. Granite: An igneous rock composed mostly of orthoclase feldspar and quartz in grains large enough to see without using a magnifier. Most granites also contain mica and amphibole Rhyolite: A felsic (light-colored) volcanic rock, the extrusive equivalent of granite. -

1922 Elizabeth T
co.rYRIG HT, 192' The Moootainetro !scot1oror,d The MOUNTAINEER VOLUME FIFTEEN Number One D EC E M BER 15, 1 9 2 2 ffiount Adams, ffiount St. Helens and the (!oat Rocks I ncoq)Ora,tecl 1913 Organized 190!i EDITORlAL ST AitF 1922 Elizabeth T. Kirk,vood, Eclttor Margaret W. Hazard, Associate Editor· Fairman B. L�e, Publication Manager Arthur L. Loveless Effie L. Chapman Subsc1·iption Price. $2.00 per year. Annual ·(onl�') Se,·ent�·-Five Cents. Published by The Mountaineers lncorJ,orated Seattle, Washington Enlerecl as second-class matter December 15, 19t0. at the Post Office . at . eattle, "\Yash., under the .-\0t of March 3. 1879. .... I MOUNT ADAMS lllobcl Furrs AND REFLEC'rION POOL .. <§rtttings from Aristibes (. Jhoutribes Author of "ll3ith the <6obs on lltount ®l!!mµus" �. • � J� �·,,. ., .. e,..:,L....._d.L.. F_,,,.... cL.. ��-_, _..__ f.. pt",- 1-� r�._ '-';a_ ..ll.-�· t'� 1- tt.. �ti.. ..._.._....L- -.L.--e-- a';. ��c..L. 41- �. C4v(, � � �·,,-- �JL.,�f w/U. J/,--«---fi:( -A- -tr·�� �, : 'JJ! -, Y .,..._, e� .,...,____,� � � t-..__., ,..._ -u..,·,- .,..,_, ;-:.. � --r J /-e,-i L,J i-.,( '"'; 1..........,.- e..r- ,';z__ /-t.-.--,r� ;.,-.,.....__ � � ..-...,.,-<. ,.,.f--· :tL. ��- ''F.....- ,',L � .,.__ � 'f- f-� --"- ��7 � �. � �;')'... f ><- -a.c__ c/ � r v-f'.fl,'7'71.. I /!,,-e..-,K-// ,l...,"4/YL... t:l,._ c.J.� J..,_-...A 'f ',y-r/� �- lL.. ��•-/IC,/ ,V l j I '/ ;· , CONTENTS i Page Greetings .......................................................................tlristicles }!}, Phoiitricles ........ r The Mount Adams, Mount St. Helens, and the Goat Rocks Outing .......................................... B1/.ith Page Bennett 9 1 Selected References from Preceding Mount Adams and Mount St. -

2018 Hazard Mitigation Plan Volume 1 Draft
Kittitas County Hazard Mitigation Plan Volume 1: Planning-Area-Wide Elements January 2019 Kittitas County HAZARD MITIGATION PLAN VOLUME 1: PLANNING-AREA-WIDE ELEMENTS JANUARY 2019 Prepared for: Kittitas County 205 West 5th Avenue, Suite 108 Ellensburg, Washington 98926 Prepared by: 8401 Arlington Blvd, Fairfax, VA 22031 Kittitas County Hazard Mitigation Plan; Volume 1—Planning-Area-Wide Elements TABLE OF CONTENTS Executive Summary ............................................................................................................ ES-1 PART 1—THE PLANNING PROCESS ................................................................................ 1 Chapter 1. Introduction to the Planning Process.................................................................1-1 1.1 Why Prepare This Plan? ................................................................................................................... 1-1 1.1.1 The Big Picture ................................................................................................................... 1-1 1.1.2 Local Concerns ................................................................................................................... 1-1 1.1.3 Purposes for Planning ......................................................................................................... 1-1 1.2 Who Will Benefit From This Plan?.................................................................................................. 1-2 1.3 How to Use This Plan ..................................................................................................................... -

Oregon Department of Human Services HEALTH EFFECTS INFORMATION
Oregon Department of Human Services Office of Environmental Public Health (503) 731-4030 Emergency 800 NE Oregon Street #604 (971) 673-0405 Portland, OR 97232-2162 (971) 673-0457 FAX (971) 673-0372 TTY-Nonvoice TECHNICAL BULLETIN HEALTH EFFECTS INFORMATION Prepared by: Department of Human Services ENVIRONMENTAL TOXICOLOGY SECTION Office of Environmental Public Health OCTOBER, 1998 CALCIUM CARBONATE "lime, limewater” For More Information Contact: Environmental Toxicology Section (971) 673-0440 Drinking Water Section (971) 673-0405 Technical Bulletin - Health Effects Information CALCIUM CARBONATE, "lime, limewater@ Page 2 SYNONYMS: Lime, ground limestone, dolomite, sugar lime, oyster shell, coral shell, marble dust, calcite, whiting, marl dust, putty dust CHEMICAL AND PHYSICAL PROPERTIES: - Molecular Formula: CaCO3 - White solid, crystals or powder, may draw moisture from the air and become damp on exposure - Odorless, chalky, flat, sweetish flavor (Do not confuse with "anhydrous lime" which is a special form of calcium hydroxide, an extremely caustic, dangerous product. Direct contact with it is immediately injurious to skin, eyes, intestinal tract and respiratory system.) WHERE DOES CALCIUM CARBONATE COME FROM? Calcium carbonate can be mined from the earth in solid form or it may be extracted from seawater or other brines by industrial processes. Natural shells, bones and chalk are composed predominantly of calcium carbonate. WHAT ARE THE PRINCIPLE USES OF CALCIUM CARBONATE? Calcium carbonate is an important ingredient of many household products. It is used as a whitening agent in paints, soaps, art products, paper, polishes, putty products and cement. It is used as a filler and whitener in many cosmetic products including mouth washes, creams, pastes, powders and lotions. -

Calcareous Soils Are Alkaline
By Mongi Zekri, Tom Obreza and Kelly Morgan alcareous soils are alkaline (pH > 7) due to the pres- ence of excess calcium carbonate (CaCO3). These soils Ccan contain from 1 percent to more than 25 percent CaCO3 by weight, with pH in the range of 7.6 to 8.4. In Florida, soil pH is usually not higher than 8.4 regardless of CaCO3 concentration. Many Florida flatwoods soils contain one or more hori- zons (layers) that are calcareous. A typical characteristic is an alkaline, loamy horizon less than 40 inches deep that can be brought to the surface during land preparation for citrus Calcareous soil in Southwest Florida planting. Increased nutritional management intensity is re- quired to successfully grow citrus on calcareous soils. Some lution of fixed P. Applied P is available to replenish the soil grove soils (e.g. ditch banks) contain considerable amounts solution for only a relatively short time before it converts to of lime rock or shell. It may not be economically justifiable less soluble forms of P. To maintain P availability to citrus to plant these sites with certain rootstocks considering the on calcareous soils, water-soluble P fertilizer should be ap- management problems and costs involved. plied on a regular, but not necessarily frequent, basis. Since Citrus fertilizer management on calcareous soils differs P accumulates in the soil, it is at least partially available as from that on non-calcareous soils because the presence of it converts to less soluble compounds with time. CaCO3 directly or indirectly affects plant availability of N, Potassium (K) P, K, Calcium (Ca), Mg, Mn, Zn, Fe and Cu. -

AVALANCHE! Yell It Out! by Dave Galvin
AVALANCHE! Yell it out! by Dave Galvin We live and recreate in the corner of the globe (do globes really have corners?) with the largest snowfall on record (world record is held by Mt. Baker Ski Area @1,140 inches or 95 feet in 1998‐‘99, followed by Mt. Rainier ‐ Paradise@1,120 inches or 93 feet in 1971‐’72). Such snow accumulation, especially in big dumps such as happened over the weekend of February 17‐19, 2012, often leads to snow sliding downhill unexpectedly. With often deadly results. Avalanche photo from Wikipedia. Lawine.jpg @ Click on this link: http://en.wikipedia.org/wiki/File:Lawine.jpg . Big Avalanches - YouTube www.youtube.com/watch?v=B0RWLxOFGLY ► 1:54► 1:54 and watch the 2‐minute clip. Turn off the annoying music that accompanies the hair‐raising video (the music implies a “yee‐ha, ride‐‘em” attitude that is disrespectful of those who have died). If your palms don’t sweat by the ending, you don’t have a pulse. Note one thing in the film clip above: how fast that snow was moving. You cannot out‐ski or out‐board the downhill momentum of an avalanche – one’s only hope, once caught, is to get off to the side, or to ride it out while staying near the surface and hope you have helpers nearby. The goal is to not get caught. More about hope below. An avalanche is “a sudden, drastic flow of snow down a slope, occurring when either natural triggers, such as loading from new snow or rain, or artificial triggers, such as snowmobilers, explosives or backcountry skiers, overload the snowpack.”1 Here in the Northwest, we typically have what are called “slab avalanches,” which react to a stress point and result in a whole section of snow (a slab) to break free and start to move downhill.