Innate Biomineralization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endoskeleton
THE EVOLUTION OF THE VERTEBRATE ENDOSKELETON AN ESSAY ON THE SIGNIFICANCE AND) MEANING OF' SEGMENTATION IN COELOMATE ANIMIALS By W1T. 13. PRIMIROSE, M.B.. Cii.B. Lately Seniior Deniionistrator of lAnatomtiy i? the (nWizversity (of Glasgozc THE EVOLUTION OF THE VERTEBRATE ENDOSKELETON WVHEN investigating the morphology of the vertebrate head, I found it necessary to discover the morphological principles on which the segmentation of the body is founded. This essay is one of the results of this investigations, and its object is to show what has determined the segmented form in vertebrate animals. It will be seen that the segmented form in vertebrates results from a condition which at no time occurs in vertebrate animals. This condition is a form of skeleton found only in animals lower in the scale of organisation than vertebrates, and has the characters of a space containing water. This space is the Coelomic Cavity. The coelomic cavity is the key to the formation of the segmented structure of the body, and is the structure that determines the vertebrate forni. The coclomic cavity is present in a well defined state from the Alnelida tuwards, so that in ainielides it is performing the functions for which a coelom was evolved. It is, however, necessary to observe the conditions prevailing anon)g still lower forms to see why a separate cavity was formed in animals, which became the means of raising them in the scale of organisation, and ultimately leading to the evolution of the vertebrate animal. I therefore propose to trace the steps in evolution by which, I presume, the coelomic cavity originated, and then show how it or its modifications have been the basis on which the whole vertebrate structure of animals is founded. -

Biology of Echinoderms
Echinoderms Branches on the Tree of Life Programs ECHINODERMS Written and photographed by David Denning and Bruce Russell Produced by BioMEDIA ASSOCIATES ©2005 - Running time 16 minutes. Order Toll Free (877) 661-5355 Order by FAX (843) 470-0237 The Phylum Echinodermata consists of about 6,000 living species, all of which are marine. This video program compares the five major classes of living echinoderms in terms of basic functional biology, evolution and ecology using living examples, animations and a few fossil species. Detailed micro- and macro- photography reveal special adaptations of echinoderms and their larval biology. (THUMBNAIL IMAGES IN THIS GUIDE ARE FROM THE VIDEO PROGRAM) Summary of the Program: Introduction - Characteristics of the Class Echinoidea phylum. spine adaptations, pedicellaria, Aristotle‘s lantern, sand dollars, urchin development, Class Asteroidea gastrulation, settlement skeleton, water vascular system, tube feet function, feeding, digestion, Class Holuthuroidea spawning, larval development, diversity symmetry, water vascular system, ossicles, defensive mechanisms, diversity, ecology Class Ophiuroidea regeneration, feeding, diversity Class Crinoidea – Topics ecology, diversity, fossil echinoderms © BioMEDIA ASSOCIATES (1 of 7) Echinoderms ... ... The characteristics that distinguish Phylum Echinodermata are: radial symmetry, internal skeleton, and water-vascular system. Echinoderms appear to be quite different than other ‘advanced’ animal phyla, having radial (spokes of a wheel) symmetry as adults, rather than bilateral (worm-like) symmetry as in other triploblastic (three cell-layer) animals. Viewers of this program will observe that echinoderm radial symmetry is secondary; echinoderms begin as bilateral free-swimming larvae and become radial at the time of metamorphosis. Also, in one echinoderm group, the sea cucumbers, partial bilateral symmetry is retained in the adult stages -- sea cucumbers are somewhat worm–like. -

ZOOLOGY Zoology 109 (2006) 164–168
ARTICLE IN PRESS ZOOLOGY Zoology 109 (2006) 164–168 www.elsevier.de/zool Mineralized Cartilage in the skeleton of chondrichthyan fishes Mason N. Dean, Adam P. Summersà Ecology and Evolutionary Biology, University of California – Irvine, 321 Steinhaus Hall, Irvine, CA 92697-2525, USA Received 10 February 2006; received in revised form 2 March 2006; accepted 3 March 2006 Abstract The cartilaginous endoskeleton of chondrichthyan fishes (sharks, rays, and chimaeras) exhibits complex arrangements and morphologies of calcified tissues that vary with age, species, feeding behavior, and location in the body. Understanding of the development, evolutionary history and function of these tissue types has been hampered by the lack of a unifying terminology. In order to facilitate reciprocal illumination between disparate fields with convergent interests, we present levels of organization in which crystal orientation/size delimits three calcification types (areolar, globular, and prismatic) that interact in two distinct skeletal types, vertebral and tessellated cartilage. The tessellated skeleton is composed of small blocks (tesserae) of calcified cartilage (both prismatic and globular) overlying a core of unmineralized cartilage, while vertebral cartilage usually contains all three types of calcification. r 2006 Elsevier GmbH. All rights reserved. Keywords: Elasmobranch skeleton; Mineralization; Calcified cartilage; Tesserae Introduction (Summers, 2000; Schaefer and Summers, 2005; Dean et al., 2006) interests in cartilaginous skeletons, we need The breadth of morphological variation of the a common language. Furthermore, the current termi- mineralized cartilage of the endoskeleton of chon- nology masks unappreciated complexity in morphology drichthyan fishes (sharks, rays, and chimaeras) has led that will fuel future research in several of these fields. -

Iron Biominerals: an Overview
IRON BIOMINERALS: AN OVERVIEW Richard B. Frankel Department of Physics California Polytechnic State University San Luis Obispo, CA 93407 INTRODUCfION Biomineralization processes, by which organisms form inorganic minerals, are broadly distributed and occur in almost every phylum of the biological world 1,2,3. There is a large diversity of minerals formed, with over 60 currently known1. The cations of the most widely occuring minerals are the divalent alkaline earths Mg, Ca, Sr and Ba. These are paired with the anions carbonate, hydroxide, oxalate, oxide, phosphate, sulfate and sulfide. Silica, hydrous silicon oxide, also occurs widely in algae (fable 1). These minerals function as exo- and endoskeletons, cation storage, lenses, gravity devices and in other roles in various organisms. IRON BIOMINERALS Minerals of iron are also known to occur in many organisms. This is due perhaps, to the important role of iron in many metabolic processes, and to the difficulty for organisms posed by the toxic products of ferrous iron oxidation by 02 together with the insolubility of ferric iron at neutral pH. Formation of iron minerals allows organisms to accumulate iron for future metabolic needs while avoiding high intracellular concentrations of ferrous iron. Other attributes of iron biominerals that are potentially useful to organisms include hardness, density and magnetism. An important class of iron biominerals are the ferric hydroxides or oxy hydroxides, which occur as amorphous, colloidal precipiates, as quasi crystalline minerals such as ferrihydrite, or as crystalline minerals such as lepidocrocite or goethite (fable 2). Amorphous iron oxy-hydroxides are found in the sheaths and stalks produced by the so-called iron bacteria, Leptothrix and Galljanella, which utilize the oxidation of ferrous ions by 02 as a source of Table 1. -

Initial Stages of Calcium Uptake and Mineral Deposition in Sea Urchin Embryos
Initial stages of calcium uptake and mineral deposition in sea urchin embryos Netta Vidavskya, Sefi Addadib, Julia Mahamidc, Eyal Shimonid, David Ben-Ezrae, Muki Shpigele, Steve Weinera, and Lia Addadia,1 aDepartment of Structural Biology, Weizmann Institute of Science, Rehovot 76100, Israel; bB-nano Ltd., Rehovot 76326, Israel; cDepartment of Molecular Structural Biology, Max Planck Institute of Biochemistry, 82152 Martinsried, Germany; dDepartment of Chemical Research Support, Weizmann Institute of Science, Rehovot 76100, Israel; and eIsrael Oceanographic and Limnological Research, National Center for Mariculture, Eilat 88112, Israel Edited by Patricia M. Dove, Virginia Polytechnic Institute and State University, Blacksburg, VA, and approved October 31, 2013 (received for review July 10, 2013) Sea urchin larvae have an endoskeleton consisting of two calcitic fluorescent signal at the spicule tip (13). The dynamics of spicule spicules. We reconstructed various stages of the formation elongation also was studied in vivo by using calcein, which la- pathway of calcium carbonate from calcium ions in sea water to beled the surface of newly formed spicule areas (7, 14). Blocking mineral deposition and integration into the forming spicules. or interfering with calcium uptake by the cells, both in vivo and Monitoring calcium uptake with the fluorescent dye calcein shows in PMC cultures, shows that for the spicule to be formed, cal- that calcium ions first penetrate the embryo and later are de- cium first has to penetrate the cells (15–17). The manner in posited intracellularly. Surprisingly, calcium carbonate deposits are which calcium is transported to the PMCs is not known (7). It is distributed widely all over the embryo, including in the primary known that the ectoderm cells take part in spicule mineraliza- mesenchyme cells and in the surface epithelial cells. -

Molecular Mechanisms of Biomineralization in Marine Invertebrates Melody S
© 2020. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2020) 223, jeb206961. doi:10.1242/jeb.206961 REVIEW Molecular mechanisms of biomineralization in marine invertebrates Melody S. Clark* ABSTRACT biodiversity (Box 1). They are also important in global carbon Much recent marine research has been directed towards cycling. For example, our recent geological history has seen the ’ understanding the effects of anthropogenic-induced environmental world s oceans become a significant net sink of anthropogenic CO2, ’ change on marine biodiversity, particularly for those animals with with the estimation that the world s continental shelves (see −1 heavily calcified exoskeletons, such as corals, molluscs and urchins. Glossary) alone absorb 0.4 Pg carbon year (see Glossary) This is because life in our oceans is becoming more challenging for (known as blue carbon) (Bauer et al., 2013). The process whereby these animals with changes in temperature, pH and salinity. In the our oceans absorb CO2 is an important buffer for future increases in future, it will be more energetically expensive to make marine atmospheric CO2 and therefore an important weapon in global skeletons and the increasingly corrosive conditions in seawater are resilience to anthropogenic climate change. Previous estimates of expected to result in the dissolution of these external skeletons. blue carbon have concentrated on calculating the carbon assimilated However, initial predictions of wide-scale sensitivity are changing as by microscopic biomineralizers that live in the water column, such we understand more about the mechanisms underpinning skeletal as the coccolithophore Emiliania huxleyi (Balch et al., 2007). production (biomineralization). These studies demonstrate the However, there is increasing recognition that the invertebrate complexity of calcification pathways and the cellular responses of macrofauna, such as echinoderms and coralline algae, play animals to these altered conditions. -

Estimating the Carbon Sink Potential of the Welsh Marine Environment Abpmer
Estimating the Carbon Sink Potential of the Welsh Marine Environment ABPmer Report No 428 Date: 21st July 2020 www.naturalresourceswales.gov.uk About Natural Resources Wales Natural Resources Wales’ purpose is to pursue sustainable management of natural resources. This means looking after air, land, water, wildlife, plants and soil to improve Wales’ well-being, and provide a better future for everyone. Evidence at Natural Resources Wales Natural Resources Wales is an evidence based organisation. We seek to ensure that our strategy, decisions, operations and advice to Welsh Government and others are underpinned by sound and quality-assured evidence. We recognise that it is critically important to have a good understanding of our changing environment. We will realise this vision by: • Maintaining and developing the technical specialist skills of our staff; • Securing our data and information; • Having a well resourced proactive programme of evidence work; • Continuing to review and add to our evidence to ensure it is fit for the challenges facing us; and • Communicating our evidence in an open and transparent way. This Evidence Report series serves as a record of work carried out or commissioned by Natural Resources Wales. It also helps us to share and promote use of our evidence by others and develop future collaborations. However, the views and recommendations presented in this report are not necessarily those of NRW and should, therefore, not be attributed to NRW. 1 www.naturalresourceswales.gov.uk Report series: NRW Evidence Report Report number: 428 Publication date: July 2020 Contract number: P21018-0072 Contractor: ABPmer Contract Manager: Vye, S. Title: Estimating the Carbon Sink Potential of the Welsh Marine Environment Author(s): Armstrong, S., Hull, S., Pearson, Z., Kay, S., Wilson, R. -

Induced Ocean Acidification for Ocean in the East China
www.nature.com/scientificreports OPEN Simulated CO2-induced ocean acidifcation for ocean in the East China: historical conditions since preindustrial time and future scenarios Han Zhang1,2 & Kuo Wang1* Since preindustrial times, as atmospheric CO2 concentration increases, the ocean continuously absorbs 2− anthropogenic CO2, reducing seawater pH and [CO]3 , which is termed ocean acidifcation. We perform Earth system model simulations to assess CO2-induced acidifcation for ocean in the East China, one of the most vulnerable areas to ocean acidifcation. By year 2017, ocean surface pH in the East China drops from the preindustrial level of 8.20 to 8.06, corresponding to a 35% rise in [H+], and reduction rate of pH becomes faster in the last two decades. Changes in surface seawater acidity largely result from CO2- induced changes in surface dissolved inorganic carbon (DIC), alkalinity (ALK), salinity and temperature, 2− among which DIC plays the most important role. By year 2300, simulated reduction in sea surface [CO]3 is 13% under RCP2.6, contrasted to 72% under RCP8.5. Furthermore, simulated results show that CO2- 2− induced warming acts to mitigate reductions in [CO]3 , but the individual efect of oceanic CO2 uptake is much greater than the efect of CO2-induced warming on ocean acidifcation. Our study quantifes ocean acidifcation induced by anthropogenic CO2, and indicates the potentially important role of accelerated CO2 emissions in projections of future changes in biogeochemistry and ecosystem of ocean in the East China. Atmospheric CO2 concentration has reached 407.44 ± 0.10 ppm (parts per million) by year 2018, increased by 46% since preindustrial time1, which is mainly due to human activities of fossil fuel burning and land use changes. -

Micropaleontological Preparation Techniques and Analyses
Les Cahiers du GEOTOP No 3 Micropaleontological preparation techniques and analyses Original version - April 1996 Second edited version - January 1999 Third edited version – June 2010 Notes prepared for students of course SCT 8245 Département des Sciences de la Terre, UQAM Prepared by Anne de Vernal, Maryse Henry and Guy Bilodeau With the collaboration of Sarah Steinhauer (2009 edition) Traduction by Olivia Gibb (2010 edition) Micropaleontological preparation techniques and analyses Table of Contents Caution Introduction 1. Sample management 1.1 Sediment core subsampling 1.2 Notebook for sediment management 2. Sample preparation techniques for carbonate microfossil analysis (foraminifera, ostracods, pteropods) 2.1. General information 2.1.1. Foraminifera 2.1.2. Ostracods 2.1.3. pteropods 2.2. Sample preparation 2.2.1. Routine techniques 2.2.2. Heavy liquid separation 2.2.3. Staining living foraminifera 2.3. Subsampling and sieving 2.4. Counting and concentration calculations 2.5. Extraction of foraminifera for stable isotope analysis 2.6. Extraction of foraminifera for 14C analysis 3. Sample preparation techniques for the analysis of coccoliths and other calcareous nannofossils analysis 3.1. General information 3.2. Sample preparation 3.3. Coccolith counting using a polarising microscope 3.4. Concentration calculations 4. Sample preparation techniques for the analysis of diatoms and other siliceous algal microfossils 4.1. General information 4.2. Sample preparation 4.3. Thin section preparation 4.4. Diatom counting using an optical microscope 4.5. Concentration calculations 5. Sample preparation techniques for palynological analysis (pollen and spores, dinoflagellate cysts, and other palynomorphs) 5.1. General information 5.1.1. Pollen, spores and other continental palynomorphs 5.1.2. -

Mollusks, Worms, Arthropods, Echinoderms
6-3.1 Compare the characteristic structures of invertebrate animals (including sponges, segmented worms, echinoderms, mollusks, and arthropods) and vertebrate animals.... Also covers: 6-1.1, 6-1.2, 6-1.5, 6-3.2 Mollusks, Worms, Arthropods, Echinoderms sections An Army of Ants! These green weaver worker ants are working together 1 Mollusks to defend their nest. These ants, and more than a 2 Segmented Worms million other species, are members of the largest and 3 Arthropods most diverse group of animals, the arthropods. In Lab Observing a Crayfish this chapter, you will be studying these animals, as 4 Echinoderms well as mollusks, worms, and echinoderms. Lab What do worms eat? Science Journal Write three animals from each animal Virtual Lab How are mollusks, group that you will be studying: mollusks, worms, arthropods, and worms, arthropods, and echinoderms echinoderms. classified? 186 Michael & Patricia Fogden/CORBIS Start-Up Activities Invertebrates Make the fol- lowing Foldable to help you organize the main characteris- Mollusk Protection tics of the four groups of com- plex invertebrates. If you’ve ever walked along a beach, espe- cially after a storm, you’ve probably seen STEP 1 Draw a mark at the midpoint of a many seashells. They come in different col- sheet of paper along the side edge. ors, shapes, and sizes. If you look closely, you Then fold the top and bottom edges will see that some shells have many rings or in to touch the midpoint. bands. In the following lab, find out what the bands tell you about the shell and the organ- ism that made it. -
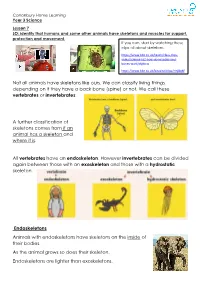
Not All Animals Have Skeletons Like Ours. We Can Classify Living Things Depending on If They Have a Back Bone (Spine) Or Not
Canonbury Home Learning Year 3 Science Lesson 7 LO: identify that humans and some other animals have skeletons and muscles for support, protection and movement. If you can, start by watching these clips all about skeletons: https://www.bbc.co.uk/teach/class-clips- video/science-ks2-how-do-muscles-and- bones-work/zfgtscw https://www.bbc.co.uk/bitesize/clips/zmj8q6f Not all animals have skeletons like ours. We can classify living things depending on if they have a back bone (spine) or not. We call these vertebrates or invertebrates: A further classification of skeletons comes from if an animal has a skeleton and where it is. All vertebrates have an endoskeleton. However invertebrates can be divided again between those with an exoskeleton and those with a hydrostatic skeleton. Endoskeletons Animals with endoskeletons have skeletons on the inside of their bodies. As the animal grows so does their skeleton. Endoskeletons are lighter than exoskeletons. Canonbury Home Learning Exoskeletons Animals with exoskeletons have their skeletons on the outside! Exoskeletons do not grow with the animal. Therefore the animal has to shed its skeleton and produce a new one! Here is a clip of a crab getting rid of his old exoskeleton: https://vimeo.com/37438364 Hydrostatic Skeletons Animals with hydrostatic skeletons don’t actually have any bones! Instead these animals have a fluid-filled compartment in their body called a coelom. Task: Create a poster or presentation (you can do this using film/power point/purple mash) about the different types of skeletons living things have. You need to find out at least 3 examples of animal that have each skeleton (endoskeleton, exoskeleton, hydrostatic skeleton) and research the pros and cons of each type of skeleton. -

Mollusks, Worms, Arthropods, and Echinoderms As Shown
22 Mollusks, Worms, Arthropods, Echinoderms hat do fiddler crabs run- ning along the beach have Win common with pill bugs and millipedes you might discover under a rock in your yard? How many mosquitoes have bitten you? These animals and more than a million other species belong to the largest, most diverse group of animals—the arthropods. In this chapter, you will learn about arthropods, as well as mollusks, worms, and echinoderms. What do you think? Science Journal Look at the picture below with a classmate. Discuss what you think this might be. Here’s a hint: It can be found in your house. Write your answer or best guess in your Science Journal. 36 ◆ C f you’ve ever walked along a beach, especially EXPLORE Iafter a storm, you’ve probably seen many seashells. They come in many different colors, shapes, and sizes. ACTIVITY If you look closely, you will see that some shells have many rings or bands. In the following activity, find out what the bands tell you about the shell and the organism that made it. Examine a clam’s shell 1. Use a hand lens to examine a clam’s shell. 2. Count the number of rings or bands on the shell. Count as number one the large, top point called the crown. 3. Compare the distances between the bands of the shell. Observe Do other students’shells have the same number of bands? Are all of the bands on your shell the same width? What do you think the bands represent, and why are some wider than others? Record your answers in your Science Journal.