Safe Shellfish Gathering and Consumption – What You Need to Know
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

AEBR 114 Review of Factors Affecting the Abundance of Toheroa Paphies
Review of factors affecting the abundance of toheroa (Paphies ventricosa) New Zealand Aquatic Environment and Biodiversity Report No. 114 J.R. Williams, C. Sim-Smith, C. Paterson. ISSN 1179-6480 (online) ISBN 978-0-478-41468-4 (online) June 2013 Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries websites at: http://www.mpi.govt.nz/news-resources/publications.aspx http://fs.fish.govt.nz go to Document library/Research reports © Crown Copyright - Ministry for Primary Industries TABLE OF CONTENTS EXECUTIVE SUMMARY ....................................................................................................... 1 1. INTRODUCTION ............................................................................................................ 2 2. METHODS ....................................................................................................................... 3 3. TIME SERIES OF ABUNDANCE .................................................................................. 3 3.1 Northland region beaches .......................................................................................... 3 3.2 Wellington region beaches ........................................................................................ 4 3.3 Southland region beaches ......................................................................................... -

Physiological Effects and Biotransformation of Paralytic
PHYSIOLOGICAL EFFECTS AND BIOTRANSFORMATION OF PARALYTIC SHELLFISH TOXINS IN NEW ZEALAND MARINE BIVALVES ______________________________________________________________ A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of Philosophy in Environmental Sciences in the University of Canterbury by Andrea M. Contreras 2010 Abstract Although there are no authenticated records of human illness due to PSP in New Zealand, nationwide phytoplankton and shellfish toxicity monitoring programmes have revealed that the incidence of PSP contamination and the occurrence of the toxic Alexandrium species are more common than previously realised (Mackenzie et al., 2004). A full understanding of the mechanism of uptake, accumulation and toxin dynamics of bivalves feeding on toxic algae is fundamental for improving future regulations in the shellfish toxicity monitoring program across the country. This thesis examines the effects of toxic dinoflagellates and PSP toxins on the physiology and behaviour of bivalve molluscs. This focus arose because these aspects have not been widely studied before in New Zealand. The basic hypothesis tested was that bivalve molluscs differ in their ability to metabolise PSP toxins produced by Alexandrium tamarense and are able to transform toxins and may have special mechanisms to avoid toxin uptake. To test this hypothesis, different physiological/behavioural experiments and quantification of PSP toxins in bivalves tissues were carried out on mussels ( Perna canaliculus ), clams ( Paphies donacina and Dosinia anus ), scallops ( Pecten novaezelandiae ) and oysters ( Ostrea chilensis ) from the South Island of New Zealand. Measurements of clearance rate were used to test the sensitivity of the bivalves to PSP toxins. Other studies that involved intoxication and detoxification periods were carried out on three species of bivalves ( P. -

Ripiro Beach
http://researchcommons.waikato.ac.nz/ Research Commons at the University of Waikato Copyright Statement: The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). The thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: Any use you make of these documents or images must be for research or private study purposes only, and you may not make them available to any other person. Authors control the copyright of their thesis. You will recognise the author’s right to be identified as the author of the thesis, and due acknowledgement will be made to the author where appropriate. You will obtain the author’s permission before publishing any material from the thesis. The modification of toheroa habitat by streams on Ripiro Beach A thesis submitted in partial fulfilment of the requirements for the degree of Master of Science (Research) in Environmental Science at The University of Waikato by JANE COPE 2018 ―We leave something of ourselves behind when we leave a place, we stay there, even though we go away. And there are things in us that we can find again only by going back there‖ – Pascal Mercier, Night train to London i Abstract Habitat modification and loss are key factors driving the global extinction and displacement of species. The scale and consequences of habitat loss are relatively well understood in terrestrial environments, but in marine ecosystems, and particularly soft sediment ecosystems, this is not the case. The characteristics which determine the suitability of soft sediment habitats are often subtle, due to the apparent homogeneity of sandy environments. -

Seashells Shells Joined Together
What shell is this? Many of the shells you see on the beach were once two Seashells shells joined together. These shells are called bivalves. by Feana Tu‘akoi Sometimes you will find them still joined, but mostly they have broken away from the other shell. Bivalves are the most common seashells washed up on our beaches. They include the shells of pipi, tuatua, oysters, mussels, and scallops. Some shells, such as limpets and pāua, are single shells. They might have small holes in the top. Other single shells, such as the Cookʼs Turban shell, are shaped like a spiral. Mussel shell Pipi shell Many of New Zealandʼs beaches are covered in shells – little shells, big shells, smooth shells, flat shells, and curly shells. Some shells are plain, and some have Cookʼs Turban shell patterns. Have you ever wondered where all these shells come from? Empty homes Almost all seashells were once homes for sea creatures. When the creatures inside the shells die or are eaten by predators, their shells are left behind. Many of the empty shells get washed up onto the beach. Limpet shell 2 3 Molluscs Shell science We often call the creatures that live in shells “shellfish” Hamish Spencer is a shell scientist. – but they are not really fish. They are part of a group of He studies shells and shellfish, and he creatures called molluscs. finds them fascinating. A mollusc has a soft body and no bones, but many Hamish has seen some unusual shellfish. Hamish Spencer molluscs have a hard shell. The shell helps to keep the “A shipworm has a body that looks like mollusc safe from predators such as seagulls, crabs, a worm, but with two small shells fish, and other, bigger, molluscs. -

1 Oct 06 IPP Final
INTRODUCTION OF NEW STOCKS INTO THE QUOTA MANAGEMENT SYSTEM ON 1 OCTOBER 2006 CONSULTATION DOCUMENT 9 August 2005 TABLE OF CONTENTS INTRODUCTION........................................................................................................1 COCKLE, PIPI AND TUATUA IN FMA10................................................................15 DEEPWATER CLAM (PZL).....................................................................................17 KNOBBED WHELK (KWH).....................................................................................25 i ii INTRODUCTION 1 In accordance with sections 17B(3) and 19(7) of the Fisheries Act 1996 (the Act), the purpose of this document is to consult on behalf of the Minister of Fisheries on those species or stocks proposed for introduction into the Quota Management System (QMS) on 1 October 2006 (refer Table 1). The Ministry of Fisheries (MFish) requests that you provide your comments on the introduction of these species or stocks into the QMS, their proposed Quota Management Areas (QMAs), fishing year, unit of measure and assessment of the legislative criteria, as outlined in this document. 2 MFish requests that you provide your written comments in response to this consultation document no later than 16 September 2005. Your comments should be in response to the proposals for the species or stocks outlined in Table 1 in relation to: · The assessment of the legislative criteria; · The QMAs, including alternative options, for each stock; · The fishing year for each stock; and · The unit -

Cockle, Pipi and Tuatua: Introduction Into the QMS
Cockle, pipi and tuatua: introduction into the QMS Most commercial cockle and pipi stocks are already in the Quota Management System (QMS). All remaining stocks of cockle, pipi and tuatua are to be introduced in October 2005. This outline summarises the main issues that have arisen when setting catch limits for these stocks. There is an opportunity for people to comment on these issues and proposed options, including the commercial and non-commercial catch limits. Cockle, pipi and tuatua are a significant non-commercial resource and are harvested extensively by customary and recreational fishers. Commercial fishing in recent years has only occurred at a small scale in Ohiwa Harbour for cockle and pipi (COC 1C, PPI 1C) and at a moderate scale in the Kaipara Harbour and other west coast beaches for tuatua (TUA 9). Information on the size of cockle, pipi and tuatua resources for any of the stocks is limited. Shellfish numbers can decline quite quickly at some beds. They are sensitive to environmental factors like flooding, habitat disturbance and pollution; and are extremely easy to harvest. Anecdotal information suggests that there is already significant non-commercial harvesting in many shellfish beds, particularly in the northern parts of the North Island. Concerns for sustainability have already resulted in a variety of management actions, such as temporary or permanent closed areas and reduced bag limits. In some areas, it is considered that current levels of harvest are already high enough, and Total Allowable Catches (TACs) based on these levels have been proposed. These TACs provide for existing recreational and customary catch, other sources of mortality due to illegal catch, and set Total Allowable Commercial Catches (TACCs) of zero. -

코끼리조개, Panopea Japonica (A
Korean J. Malacol. 30(4): 303-309 2014 http://dx.doi.org/10.9710/kjm.2014.30.4.303 코끼리조개, Panopea japonica (A. Adams) 의 수정란 발생과 유생 성장 남명모, 이주, 김미경, 김재원1, 김영대 국립수산과학원 동해수산연구소, 1강원도립대학 해양생명과학과 Development and growth in fertilized eggs and larvae of the Japanese geoduck, Panopea japonica reared in the laboratory Myung-Mo Nam, Chu Lee, MeeKyung Kim, Jae Won Kim1 and Young Dae Kim East Sea Fisheries Research Institute NFRDI, Gangneung 210-861, Korea 1Dept. Marine Life Science and Aquaculture, Gangwon Provincial College, Gangneung 210-804, Korea ABSTRACT The development of Japanese geoduck, Panopea japonica, grown under culture conditions, has been examined through the morphological characteristics in fertilized egg, larvae and juvenile. Gametes were stripped from ripe broodstock and placed into two separate containers. Eggs were washed through a 40 μm sieve and fertilized with dilute sperm solution. Developing larvae were maintained at 19 ± 1℃. Fertilized eggs with 81.6 μm diameter developed to trochophores within 14 h and to D-stage larvae (116 μm shell length) within 27 h. Larvae were spontaneously settled at shell length of 311 μm after 20 days. The hatching from fertilized eggs and larval rearing were normally available in 18.5-21.5℃, and the growth was good in a cashmilon substrate, as well as sand. After rearing of day 108 from metamorphosis, the shell length of juvenile P. japonica reached 13 mm, and growth rate of shell length of the juvenile was 117.5 μm/d. Key words: Geoduck, Development, Growth, Fertilized egg, Larvae, Juvenile, Panopea japonica 서 론 (Min et al., 2004) 로서 성숙한 개체는 식용가식부가 55% 내 외로 많고 향과 맛이 좋을 뿐만 아니라 단백질이 풍부하여 양 코끼리조개 (Panopea japonica, Japanese geoduck) 는 식 대상종으로 개발 가치가 아주 큰 종이다. -

Distribution and Abundance of Toheroa (Paphies Ventricosa) and Tuatua (P
Distribution and abundance of toheroa (Paphies ventricosa) and tuatua (P. subtriangulata) at Ninety Mile Beach in 2010 and Dargaville Beach in 2011 New Zealand Fisheries Assessment Report 2013/39 J. Williams, H. Ferguson, I. Tuck ISSN 1179-5352 (online) ISBN 978-0-478-41436-3 (online) May 2013 Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries websites at: http://www.mpi.govt.nz/news-resources/publications.aspx http://fs.fish.govt.nz go to Document library/Research reports © Crown Copyright - Ministry for Primary Industries. TABLE OF CONTENTS Executive Summary ...................................................................................................................................................... 1 Objectives ..................................................................................................................................................................... 2 1 INTRODUCTION ................................................................................................................................................ 3 1.1 Toheroa and tuatua ....................................................................................................................................... 3 1.2 Toheroa fisheries and surveys ..................................................................................................................... -

Genetic Variation in New Zealand Abalone, Haliotis Iris
Genetic variation in New Zealand abalone, Haliotis iris A thesis submitted in partial fulfillment of the of the requirements for the Degree of Doctor of Philosophy in Biological Sciences at the University of Canterbury by Margaret Will University of Canterbury Christchurch, New Zealand 2009 Table of Contents ABSTRACT ...............................................................................................................................1 1. INTRODUCTION..................................................................................................................3 GENE FLOW.............................................................................................................................3 ABALONE ................................................................................................................................7 Systematics .........................................................................................................................9 Assessing genetic structure...............................................................................................11 Genetic structure of abalone.............................................................................................12 NEW ZEALAND ABALONE......................................................................................................20 Aims .................................................................................................................................22 2. GENETIC STRUCTURE ACROSS COOK STRAIT.........................................................25 -
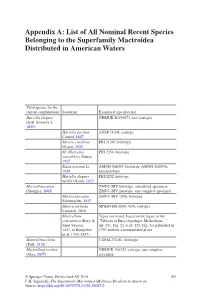
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype; -
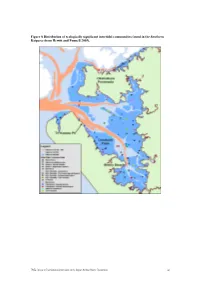
Figure 8 Distribution of Ecologically Significant Intertidal Communities Found in the Southern Kaipara (From Hewitt and Funnell 2005)
Figure 8 Distribution of ecologically significant intertidal communities found in the Southern Kaipara (from Hewitt and Funnell 2005). TP354: Review of Environmental Information on the Kaipara Harbour Marine Environment 21 Figure 9 Interpolated plots of the distribution of total numbers of individuals (A), number of taxa (B), and number of orders (C) found in the cores taken from the intertidal sites (from Hewitt and Funnell 2005). A B C TP354: Review of Environmental Information on the Kaipara Harbour Marine Environment 22 Figure 10 Distribution of subtidal epibenthic habitats found in the Southern Kaipara (from Hewitt and Funnell 2005). TP354: Review of Environmental Information on the Kaipara Harbour Marine Environment 23 Figure 11 Distribution of ecologically significant subtidal communities found in the Southern Kaipara (from Hewitt and Funnell 2005). TP354: Review of Environmental Information on the Kaipara Harbour Marine Environment 24 Figure 12 Interpolated plots of the distribution of total numbers of individuals (A), number of taxa (B), and number of orders (C) found in grabs taken from the subtidal sites (from Hewitt and Funnell 2005). A B C TP354: Review of Environmental Information on the Kaipara Harbour Marine Environment 25 3.2.2 Northern Kaipara Harbour The intertidal and subtidal areas of the northern Kaipara are influenced by several relatively large rivers including the Arapaoa River, Otamatea River, Oruawharo River and Wairoa River (Figure 1). Compared to the southern Kaipara, the northern Kaipara has been studied in far less spatial detail. Several studies have focused on benthic communities within discrete locations (e.g. the Otamatea River; see Robertson et al.