Cumulative Report About Obtained Results at the Dissertation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 2 Incandescent Light Bulb
Lamp Contents 1 Lamp (electrical component) 1 1.1 Types ................................................. 1 1.2 Uses other than illumination ...................................... 2 1.3 Lamp circuit symbols ......................................... 2 1.4 See also ................................................ 2 1.5 References ............................................... 2 2 Incandescent light bulb 3 2.1 History ................................................. 3 2.1.1 Early pre-commercial research ................................ 4 2.1.2 Commercialization ...................................... 5 2.2 Tungsten bulbs ............................................. 6 2.3 Efficacy, efficiency, and environmental impact ............................ 8 2.3.1 Cost of lighting ........................................ 9 2.3.2 Measures to ban use ...................................... 9 2.3.3 Efforts to improve efficiency ................................. 9 2.4 Construction .............................................. 10 2.4.1 Gas fill ............................................ 10 2.5 Manufacturing ............................................. 11 2.6 Filament ................................................ 12 2.6.1 Coiled coil filament ...................................... 12 2.6.2 Reducing filament evaporation ................................ 12 2.6.3 Bulb blackening ........................................ 13 2.6.4 Halogen lamps ........................................ 13 2.6.5 Incandescent arc lamps .................................... 14 2.7 Electrical -

The High Performance Home Manual
THE HIGH PERFORMANCE HOME MANUAL THE HIGH PERFORMANCE HOME MANUAL This document, along with the detailed drawings, is presented to offer our clients a roadmap in developing an "High Performance" home. This is not necessarily a "Green" home and does not take into consideration air-quality. It focuses on resource efficiency, energy efficiency, water conservation, and "providing for the future"; all of which are a part of a “green” home design, but not exhaustive. In some instances, we have provided “preferred” options as well as less costly means of attaining a near "High Performance" home. IMPORTANT: It is important to note that this information (these parameters) and the related detailed drawings are specifically for homes that are to be built in Southwest Texas (primarily the "hill country" type climate). The Climate Zone is three (3). Site Design Features: • Heat Mitigation: a. Shade hardscape (drives, walks, etc.) with shade trees or such. b. Utilize turf pavers for drive and/or walks, patios, etc. Resource Efficiency: • Drip edge (eaves and gables): a. Minimizes wicking and water distribution off roof material, decking, and fascia. • Roof Water Discharge: a. Provide gutters and downspout system with splash blocks (or such) to carry water a minimum of five feet from foundation (or utilize water harvesting system – see below). • Finish Grade: a. Provide a minimum fall of six inches for each ten feet from edge of building. • Flashing (galvanized metal): a. Flash roof valleys. b. Flash deck/balcony to building intersections. c. Flash at roof-to-wall intersections and roof-to-chimney intersections. d. Provide a drip cap above windows and doors that are not flashed or protected by coverings like pent roofs or are recessed in the exterior wall at least 24 inches. -

357 Final PART 1/3 COMMISSION STAFF
EUROPEAN COMMISSION Brussels, 1.10.2019 SWD(2019) 357 final PART 1/3 COMMISSION STAFF WORKING DOCUMENT IMPACT ASSESSMENT Accompanying the document COMMISSION REGULATION (EU) .../... laying down ecodesign requirements for light sources and separate control gears pursuant to Directive 2009/125/EC of the European Parliament and of the Council and repealing Commission Regulations (EC) No 244/2009, (EC) No 245/2009 and (EU) No 1194/2012 and COMMISSION DELEGATED REGULATION (EU) .../... supplementing Regulation (EU) 2017/1369 of the European Parliament and of the Council with regard to energy labelling of light sources and repealing Commission Delegated Regulation (EU) No 874/2012 {C(2019) 1805 final} - {C(2019) 2121 final} - {SEC(2019) 340 final} - {SWD(2019) 358 final} EN EN Table of contents TABLE OF CONTENTS ..................................................................................................................................................................................................... 1 1. INTRODUCTION: POLITICAL AND LEGAL CONTEXT .................................................................................................................................. 2 1.1. Benefits of Ecodesign and Energy Labelling .......................................................................................................................................... 3 1.2. Legal framework ................................................................................................................................................................................... -

An Overview Contents
Neon An overview Contents 1 Overview 1 1.1 Neon .................................................. 1 1.1.1 History ............................................ 1 1.1.2 Isotopes ............................................ 2 1.1.3 Characteristics ........................................ 2 1.1.4 Occurrence .......................................... 3 1.1.5 Applications .......................................... 3 1.1.6 Compounds .......................................... 4 1.1.7 See also ............................................ 4 1.1.8 References .......................................... 4 1.1.9 External links ......................................... 5 2 Isotopes 6 2.1 Isotopes of neon ............................................ 6 2.1.1 Table ............................................. 6 2.1.2 References .......................................... 6 3 Miscellany 8 3.1 Neon sign ............................................... 8 3.1.1 History ............................................ 8 3.1.2 Fabrication .......................................... 9 3.1.3 Applications .......................................... 12 3.1.4 Images of neon signs ..................................... 13 3.1.5 See also ............................................ 13 3.1.6 References .......................................... 13 3.1.7 Further reading ........................................ 14 3.1.8 External links ......................................... 14 3.2 Neon lamp ............................................... 14 3.2.1 History ........................................... -

Artificial Optical Radiation
Cyan 100% Magenta 76% Yellow 0 Black 27% Guidance for Employers on the Control of Artifi cial Optical Radiation at Work Regulations 2010 Our vision: A national culture where all commit to safe and healthy workplaces and the safe and sustainable management of chemicals Contents Introduction 2 General background information 4 Purpose of the Regulations 5 Requirement of the Control of Artifi cial Optical Radiation at Work Regulations 2010 7 Annexes 14 Annex (A): List of safe light sources 14 Annex (B): Work activities which generate hazardous levels of intense light and the appropriate control measures for them 15 Annex (C): Hierarchical approach for more complex risk assessments 20 Annex (D): List of key safety signs 23 Annex (E): Personal Protective Equipment (PPE) 23 Annex (F): List of less common issues that may be relevant to your business 25 Annex (G): Sources of further Information 25 Published in 2010 by the Health and Safety Authority, The Metropolitan Building, James Joyce Street, Dublin 1. ©All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the Health and Safety Authority. Introduction Artifi cial optical radiation exists in most It applies to all undertakings where workers workplaces. Many present little or no risk of may be exposed to artifi cial optical radiation. causing injury or ill health and some allow work It aims to lead users through a logical path for activities to be carried out safely. -

How Do We Get Light from Matter: the Origin of Emis- Sion Lines
1 How Do We Get Light from Matter: The Origin of Emis- sion Lines ORGANIZATION • Pre-Lab: \Origins of Lines" • Mode: inquiry, groups of 2 • Grading: lab notes and post-lab questions • Safety: no special requirements GOAL: To examine the relationship between electron transitions and emission of light from various sources. I: BACKGROUND See all pre-lab exercises to this point for background material. II: EXERCISES You will be moving between different stations to observe the profile of light emitted from different type of sources of light. You'll be observing these sources of light with your eye, with an Ocean Optics spectrophotometer using a fiber optic cable and a visible light detector, and/or with a handheld spectroscope, using your eye as the visible light detector. Please take a moment to read through the introductory material|it will reduce frustration and help you organize and plan your work. Part A: Stations You can move between the stations in any order, but here is the list of light sources you will be observing. c 2016 Advanced Instructional Systems, Inc. dba WebAssign, a Virginia Corporation and Cengage Company, and San Francisco State University Department of Chemistry and Biochemistry Faculty, Fall 2016 Update by Nancy Gerber 2 • Station A: Everyday Sources of Light|light bulbs, CFLs (compact fluorescent lamps), overhead fluorescent lights, sunlight, light emitted from a computer screen. • Station B: Semiconductor Sources of Light|light emitting diodes (LEDs). • Station C: Atomic Sources of Light|gas-discharge lamps As you observe the profiles of these different sources of light, consider the following questions. -

HANDBOOK of OPTICAL FILTERS for FLUORESCENCE MICROSCOPY
CHROMA TECHNOLOGY CORP HANDBOOK of OPTICAL FILTERS for FLUORESCENCE MICROSCOPY by JAY REICHMAN HB2.0/May 2012 CHROMA TECHNOLOGY CORP AN INTRODUCTION TO HANDBOOK of FLUORESCENCE MICROSCOPY 2 OPTICAL FILTERS Excitation and emission spectra for FLUORESCENCE Brightness of the fluorescence signal The fluorescence microscope MICROSCOPY Types of filters used in fluorescence microscopy The evolution of the fluorescence microscope by JAY REICHMAN A GENERAL DISCUSSION OF OPTICAL FILTERS 8 Terminology Available products Colored filter glass Thin-film coatings Acousto-optical filters Liquid Crystal Tunable Filters DESIGNING FILTERS FOR FLUORESCENCE MICROSCOPY 14 Image Contrast Fluorescence spectra Light sources Detectors Beamsplitters Optical quality Optical quality parameters Optical quality requirements for wide-field microscopy FILTER SETS FOR SUB-PIXEL REGISTRATION 24 FILTERS FOR CONFOCAL MICROSCOPY 25 Optical quality requirements Nipkow-disk scanning Laser scanning Spectral requirements Nipkow-disk scanning Laser scanning FILTERS FOR MULTIPLE PROBE APPLICATIONS 29 REFERENCES 30 GLOSSARY 31 Fluorescence microscopy requires optical filters that have demanding spectral and physical characteristics. These performance requirements can vary greatly depending on the specific type of microscope and the specific application. Although they are relatively minor components of a complete microscope system, optimally designed filters can produce quite dramatic benefits, so it is useful to have a working knowledge of the principles of optical filtering as applied to fluorescence microscopy. This guide is a compilation of the principles and know-how that the engineers at Chroma Technology Corp use to design filters for a variety of fluorescence microscopes and applications, including wide-field microscopes, confocal microscopes, and applications involving simultaneous detection of multiple fluorescent probes. -

Primary ENGLISH Boek
e-learning for primary teachers A step-by-step guide to improving teaching and learning in your classroom This Comenius multilateral project has been funded with the support from the European Commission / Project number 517726-LLP-1-2011-1-BE-COMENIUS-CMP TACCLE2 e-learning for primary teachers A step-by-step guide to improving teaching and learning in your classroom Jenny hughes, nicholas daniels, Editors Jens vermeersch, Project coordinator Fernando Albuquerque Costa, Jan Bierweiler, Linda Castañeda, Nicholas Daniels, Kylene De Angelis, Giulio Gabbianelli, Bruna Durazzi, Mattia Crivellini, Koen DePryck, Gabriela Grosseck, Isabel Gutiérrez Porlán, Jenny Hughes, Laura Malita, Cidalia Marques, Paz Prendes, Pedro Reis, Carla Rodriguez, Mar Sánchez, Carine Schepers, Katleen Vanden Driessche, Authors tACCLe2 - e-LeArning for primAry teachers A step-by-step guide to improving teaching and learning in your classroom Brussels, go! onderwijs van de vlaamse gemeenschap, 2013 If you have any questions regarding this book or the project from which it originated: Jens Vermeersch GO! Onderwijs van de Vlaamse Gemeenschap Internationalisation department Brussels E-mail: [email protected] Jenny Hughes, Nicholas Daniels [Eds.] 76 pp. – 29,7 cm. D/2014/8479/001 ISBN 9789078398134 The editing of this book was finished on 28th of February 2013. Cover-design and layout: Bart Vliegen (www.watchitproductions.be) projeCt website: www.taccle2.eu This Comenius multilateral project has been funded with support from the European Commission Project NUMBER: 517726-LLP-1-2011-1-BE-COMENIUS-CMP. This book reflects the views only of the authors, and the Commission cannot be held responsible for any use that may be made of the information contained therein. -

LED Light Meter
R8140 LED Light Meter Instruction Manual www.REEDINSTRUMENTS.com Find Quality Products Online at: www.GlobalTestSupply.com [email protected] Table of Contents Introduction ................................................................................................ 3 Product Quality ........................................................................................... 3 Safety ......................................................................................................... 3 Features ...................................................................................................... 3 Included ...................................................................................................... 3 Specifications ............................................................................................. 4 Instrument Description ............................................................................... 5 Display Description .................................................................................... 5 Application Notes and User Tips ................................................................ 6 Protective Cap ...................................................................................... 6 Adjustable Sensor ................................................................................ 6 Operating Instructions .............................................................................6-8 Power On/Off (Automatic Zero Adjustment) .......................................... 6 Unit of Measure -

Nightup Castelldefels Materials I Activitats Per Docents
NIGHTUP CASTELLDEFELS MATERIALS I ACTIVITATS PER DOCENTS PER QUÈ CONTAMINACIÓ LUMÍNICA? Segurament has anat algun dia al camp o a la muntanya, lluny de les grans ciutats, i t’ha sorprès la gran quantitat d’estrelles que podies observar en el cel durant la nit. Però si mires el cel des d’una ciutat te n’adones que pràcticament no en pots veure cap. Aquest és l’efecte més evident de la contaminació lumínica, que és deguda a la llum artificial que hi ha per tot arreu a les ciutats i altres indrets. Les conseqüències de la contaminació lumínica van més enllà de no poder veure les estrelles: canvis en el comportament dels animals, desordres del son, diferents malalties, desequilibris dels ecosistemes... Tot i això, entre els diferents problemes mediambientals que patim actualment, el de la contaminació lumínica és un dels més senzills de solucionar. Per entendre millor aquest problema, l’ICFO està coordinant el projecte NightUp Castelldefels, un experiment col·laboratiu per investigar la importància del color en la contaminació lumínica. Els participants han de fer fotografies a fonts de llum artificial que troben al carrer a través de la web app del projecte (nightup.icfo.eu). Després, aquestes fotografies seran analitzades amb l’objectiu de realitzar un mapa del color de la il·luminació artificial que pugui ajudar els científics a entendre millor l’impacte de la contaminació lumínica. ICFO I CIÈNCIA CIUTADANA L’ICFO és un centre de recerca que té com a objectiu fer avançar els límits del coneixement de la fotònica, la ciència i la tecnologia de la llum i que està situat al Parc Mediterrani de la Tecnologia de Castelldefels. -
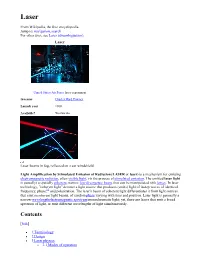
Lasers As Weapons Y 9 Fictional Predictions Y 10 See Also Y 11 Notes and References Y 12 Further Reading Y 13 External Links
Laser From Wikipedia, the free encyclopedia Jump to: navigation, search For other uses, see Laser (disambiguation). Laser United States Air Force laser experiment Inventor Charles Hard Townes Launch year 1960 Available? Worldwide Laser beams in fog, reflected on a car windshield Light Amplification by Stimulated Emission of Radiation (LASER or laser) is a mechanism for emitting electromagnetic radiation, often visible light, via the process of stimulated emission. The emitted laser light is (usually) a spatially coherent, narrow low-divergence beam, that can be manipulated with lenses. In laser technology, "coherent light" denotes a light source that produces (emits) light of in-step waves of identical frequency, phase,[1] and polarization. The laser's beam of coherent light differentiates it from light sources that emit incoherent light beams, of random phase varying with time and position. Laser light is generally a narrow-wavelengthelectromagnetic spectrum monochromatic light; yet, there are lasers that emit a broad spectrum of light, or emit different wavelengths of light simultaneously. Contents [hide] y 1 Terminology y 2 Design y 3 Laser physics o 3.1 Modes of operation 3.1.1 Continuous wave operation 3.1.2 Pulsed operation 3.1.2.1 Q-switching 3.1.2.2Modelocking 3.1.2.3 Pulsed pumping y 4 History o 4.1 Foundations o 4.2 Maser o 4.3 Laser o 4.4 Recent innovations y 5 Types and operating principles o 5.1 Gas lasers 5.1.1 Chemical lasers 5.1.2Excimer lasers o 5.2 Solid-state lasers 5.2.1Fiber-hosted lasers 5.2.2 Photonic crystal lasers 5.2.3 Semiconductor lasers o 5.3 Dye lasers o 5.4 Free electron lasers o 5.5 Exotic laser media y 6 Uses o 6.1 Examples by power o 6.2 Hobby uses y 7 Laser safety y 8 Lasers as weapons y 9 Fictional predictions y 10 See also y 11 Notes and references y 12 Further reading y 13 External links Terminology From left to right: gamma rays, X-rays, ultraviolet rays, visible spectrum, infrared, microwaves, radio waves. -

HANDBOOK of OPTICAL FILTERS for FLUORESCENCE MICROSCOPY
CHROMA TECHNOLOGY CORP HANDBOOK of OPTICAL FILTERS for FLUORESCENCE MICROSCOPY by JAY REICHMAN HB2.0/June 2017 CHROMA TECHNOLOGY CORP AN INTRODUCTION TO HANDBOOK of FLUORESCENCE MICROSCOPY 2 OPTICAL FILTERS Excitation and emission spectra for FLUORESCENCE Brightness of the fluorescence signal The fluorescence microscope MICROSCOPY Types of filters used in fluorescence microscopy The evolution of the fluorescence microscope by JAY REICHMAN A GENERAL DISCUSSION OF OPTICAL FILTERS 8 Terminology Available products Colored filter glass Thin-film coatings Acousto-optical filters Liquid Crystal Tunable Filters DESIGNING FILTERS FOR FLUORESCENCE MICROSCOPY 14 Image Contrast Fluorescence spectra Light sources Detectors Beamsplitters Optical quality Optical quality parameters Optical quality requirements for wide-field microscopy FILTER SETS FOR SUB-PIXEL REGISTRATION 24 FILTERS FOR CONFOCAL MICROSCOPY 25 Optical quality requirements Nipkow-disk scanning Laser scanning Spectral requirements Nipkow-disk scanning Laser scanning FILTERS FOR MULTIPLE PROBE APPLICATIONS 29 REFERENCES 30 GLOSSARY 31 Fluorescence microscopy requires optical filters that have demanding spectral and physical characteristics. These performance requirements can vary greatly depending on the specific type of microscope and the specific application. Although they are relatively minor components of a complete microscope system, optimally designed filters can produce quite dramatic benefits, so it is useful to have a working knowledge of the principles of optical filtering as applied to fluorescence microscopy. This guide is a compilation of the principles and know-how that the engineers at Chroma Technology Corp use to design filters for a variety of fluorescence microscopes and applications, including wide-field microscopes, confocal microscopes, and applications involving simultaneous detection of multiple fluorescent probes.