Not As Widespread As Thought: Integrative Taxonomy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amphibians in Zootaxa: 20 Years Documenting the Global Diversity of Frogs, Salamanders, and Caecilians
Zootaxa 4979 (1): 057–069 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Review ZOOTAXA Copyright © 2021 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4979.1.9 http://zoobank.org/urn:lsid:zoobank.org:pub:972DCE44-4345-42E8-A3BC-9B8FD7F61E88 Amphibians in Zootaxa: 20 years documenting the global diversity of frogs, salamanders, and caecilians MAURICIO RIVERA-CORREA1*+, DIEGO BALDO2*+, FLORENCIA VERA CANDIOTI3, VICTOR GOYANNES DILL ORRICO4, DAVID C. BLACKBURN5, SANTIAGO CASTROVIEJO-FISHER6, KIN ONN CHAN7, PRISCILLA GAMBALE8, DAVID J. GOWER9, EVAN S.H. QUAH10, JODI J. L. ROWLEY11, EVAN TWOMEY12 & MIGUEL VENCES13 1Grupo Herpetológico de Antioquia - GHA and Semillero de Investigación en Biodiversidad - BIO, Universidad de Antioquia, Antioquia, Colombia [email protected]; https://orcid.org/0000-0001-5033-5480 2Laboratorio de Genética Evolutiva, Instituto de Biología Subtropical (CONICET-UNaM), Facultad de Ciencias Exactas Químicas y Naturales, Universidad Nacional de Misiones, Posadas, Misiones, Argentina [email protected]; https://orcid.org/0000-0003-2382-0872 3Unidad Ejecutora Lillo, Consejo Nacional de Investigaciones Científicas y Técnicas - Fundación Miguel Lillo, 4000 San Miguel de Tucumán, Argentina [email protected]; http://orcid.org/0000-0002-6133-9951 4Laboratório de Herpetologia Tropical, Universidade Estadual de Santa Cruz, Departamento de Ciências Biológicas, Rodovia Jorge Amado Km 16 45662-900 Ilhéus, Bahia, Brasil [email protected]; https://orcid.org/0000-0002-4560-4006 5Florida Museum of Natural History, University of Florida, 1659 Museum Road, Gainesville, Florida, 32611, USA [email protected]; https://orcid.org/0000-0002-1810-9886 6Laboratório de Sistemática de Vertebrados, Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Av. -

Stream Frogs, Mannophryne Trinitatis (Dendrobatidae): an Example of Anti-Predator Behaviour
HERPETOLOGICAL JOURNAL, Vol. 11, pp. 91-100 (2001) SELECTION OF TAD POLE DEPOSITION SITES BY MALE TRINIDADIAN STREAM FROGS, MANNOPHRYNE TRINITATIS (DENDROBATIDAE): AN EXAMPLE OF ANTI-PREDATOR BEHAVIOUR J. R. DOWNIE, S. R. LIVINGSTONE AND J. R. CORMACK Division of Environmental and Evolutionary Biology, Institute of Biomedical & Life Sciences, University of Glasgow, Scotland, UK Trinidad's only dendrobatid frog, Mannophryne (=Colostethus) trinitatis, lives by the small streams draining the slopes of the Northern Range mountains and at Tamana Hill in the Central Range. Adults are often very abundant, but tadpoles are found patchily in the streams. In the absence of two potential predators - the fish Rivulus hartii and shrimps of the genus Macrobrachium - tadpoles are abundant in pools. Where the predators are present, tadpoles are uncommon or absent. Tadpoles may also be found in small, isolated bodies of water at some distance from streams. Males carrying tadpoles retained them for 3-4 days, in the absence of suitable pools. When presented with a choice of pools, males preferred to deposit their tadpoles in pools lacking predators. There were differences in behaviour between males fromthe northern and southern slopes of the Northern Range. For example, north coast males deposited tadpoles in pools containing other conspecific tadpoles in preference to empty pools, whereas males from southern slopes made the opposite choice. When presented only with pools containing predators (i.e. shrimps or fish), north coast males shed their tadpoles in damp leaf litter rather than in the pools, while males from the southern slopes deposited tadpoles in pools with shrimps - predators uncommon in the southern slopes streams. -

Catalogue of the Amphibians of Venezuela: Illustrated and Annotated Species List, Distribution, and Conservation 1,2César L
Mannophryne vulcano, Male carrying tadpoles. El Ávila (Parque Nacional Guairarepano), Distrito Federal. Photo: Jose Vieira. We want to dedicate this work to some outstanding individuals who encouraged us, directly or indirectly, and are no longer with us. They were colleagues and close friends, and their friendship will remain for years to come. César Molina Rodríguez (1960–2015) Erik Arrieta Márquez (1978–2008) Jose Ayarzagüena Sanz (1952–2011) Saúl Gutiérrez Eljuri (1960–2012) Juan Rivero (1923–2014) Luis Scott (1948–2011) Marco Natera Mumaw (1972–2010) Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(1) [Special Section]: 1–198 (e180). Catalogue of the amphibians of Venezuela: Illustrated and annotated species list, distribution, and conservation 1,2César L. Barrio-Amorós, 3,4Fernando J. M. Rojas-Runjaic, and 5J. Celsa Señaris 1Fundación AndígenA, Apartado Postal 210, Mérida, VENEZUELA 2Current address: Doc Frog Expeditions, Uvita de Osa, COSTA RICA 3Fundación La Salle de Ciencias Naturales, Museo de Historia Natural La Salle, Apartado Postal 1930, Caracas 1010-A, VENEZUELA 4Current address: Pontifícia Universidade Católica do Río Grande do Sul (PUCRS), Laboratório de Sistemática de Vertebrados, Av. Ipiranga 6681, Porto Alegre, RS 90619–900, BRAZIL 5Instituto Venezolano de Investigaciones Científicas, Altos de Pipe, apartado 20632, Caracas 1020, VENEZUELA Abstract.—Presented is an annotated checklist of the amphibians of Venezuela, current as of December 2018. The last comprehensive list (Barrio-Amorós 2009c) included a total of 333 species, while the current catalogue lists 387 species (370 anurans, 10 caecilians, and seven salamanders), including 28 species not yet described or properly identified. Fifty species and four genera are added to the previous list, 25 species are deleted, and 47 experienced nomenclatural changes. -

Amphibia: Anura) Da Reserva Catuaba E Seu Entorno, Acre, Amazônia Sul-Ocidental
Efeitos da sucessão florestal sobre a anurofauna (Amphibia: Anura) da Reserva Catuaba e seu entorno, Acre, Amazônia sul-ocidental Vanessa M. de Souza 1; Moisés B. de Souza 2 & Elder F. Morato 2 1 Avenida Assis Chateaubriand 1170, ap. 304, Edifício Azul, Setor Oeste, 74130-011 Goiânia, Goiás, Brasil. E-mail: [email protected] 2 Departamento de Ciências da Natureza, Universidade Federal do Acre. Rodovia BR-364, km 04, Distrito Industrial, 69915-900 Rio Branco, Acre, Brasil. E-mail: [email protected]; [email protected] ABSTRACT. Effect of the forest succession on the anurans (Amphibia(Amphibia: Anura) of the Reserve Catuaba and its peripheryy, Acree, southwestern Amazonia. The objective of this work it was verify the abundance, richness, and the anuran composition in plots of vegetation of different succession stages in a forest and the matrix that surrounds it, of Acre (10º04’S, 67º37’W). The sampling was carried out between August 2005 and April 2006 in twelve plots located in three different sites in the forest. In each site four kinds of environments were chosen: primary forest (wood), secondary forest (capoeira), periphery (matrix) and secondary forest (succession). A total of twenty-seven species distributed in seven families was found. Greater abundance was registered in the plots of matrix two and capoeira three and the least in succession one. The richness was greater in matrix two, with the greater number of exclusive species. The abundance of anurans correlated significantly, with the average circum- ference at the breast height of the trees of the plots. The richness however correlated only marginally, with this structural feature. -

Sexual Maturity and Sexual Dimorphism in a Population of the Rocket-Frog Colostethus Aff
Tolosa et al. Actual Biol Volumen 37 / Número 102, 2014 Sexual maturity and sexual dimorphism in a population of the rocket-frog Colostethus aff. fraterdanieli (Anura: Dendrobatidae) on the northeastern Cordillera Central of Colombia Madurez y dimorfismo sexual de la ranita cohete Colostethus aff. fraterdanieli (Anura: Dendrobatidae) en una población al este de la Cordillera Central de Colombia Yeison Tolosa1, 2, *, Claudia Molina-Zuluaga1, 4,*, Adriana Restrepo1, 5, Juan M. Daza1, ** Abstract The minimum size of sexual maturity and sexual dimorphism are important life history traits useful to study and understand the population dynamics of any species. In this study, we determined the minimum size at sexual maturity and the existence of sexual dimorphism in a population of the rocket-frog, Colostethus aff. fraterdanieli, by means of morphological and morphometric data and macro and microscopic observation of the gonads. Females attained sexual maturity at 17.90 ± 0.1 mm snout-vent length (SVL), while males attained sexual maturity at 16.13 ± 0.06 mm SVL. Females differed from males in size, shape and throat coloration. Males were smaller than females and had a marked and dark throat coloration that sometimes extended to the chest, while females lacked this characteristic, with a throat either immaculate or weakly pigmented. In this study, we describe some important aspects of the reproductive ecology of a population of C. aff. fraterdanieli useful as a baseline for other more specialized studies. Key words: Amphibian, Andes, gonads, histology, morphometry, reproduction Resumen El tamaño mínimo de madurez sexual y el dimorfismo sexual son importantes características de historia de vida, útiles para estudiar y comprender la dinámica poblacional de cualquier especie. -

Two New Endangered Species of Anomaloglossus (Anura: Aromobatidae) from Roraima State, Northern Brazil
Zootaxa 3926 (2): 191–210 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2015 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3926.2.2 http://zoobank.org/urn:lsid:zoobank.org:pub:BCA3901A-DF07-4FAF-8386-C24649557313 Two new endangered species of Anomaloglossus (Anura: Aromobatidae) from Roraima State, northern Brazil ANTOINE FOUQUET1,2,8, SERGIO MARQUES SOUZA2, PEDRO M. SALES NUNES2,3, PHILIPPE J. R. KOK4,5, FELIPE FRANCO CURCIO2,6, CELSO MORATO DE CARVALHO7, TARAN GRANT2 & MIGUEL TREFAUT RODRIGUES2 1CNRS Guyane USR3456, Immeuble Le Relais, 2 Avenue Gustave Charlery, 97300, Cayenne, French Guiana 2Universidade de São Paulo, Instituto de Biociências, Departamento de Zoologia, Caixa Postal 11.461,CEP 05508-090, São Paulo, SP, Brazil 3Universidade Federal de Pernambuco, Centro de Ciências Biológicas, Departamento de Zoologia, Av. Professor Moraes Rego, s/n. Cidade Universitária CEP 50670-901, Recife, PE, Brazil 4Biology Department, Amphibian Evolution Lab, Vrije Universiteit Brussel, 2 Pleinlaan, B- 1050 Brussels, Belgium 5Department of Recent Vertebrates, Royal Belgian Institute of Natural Sciences, 29 rue Vautier, B- 1000 Brussels, Belgium 6Universidade Federal de Mato Grosso, Instituto de Biociências, Departamento de Biologia e Zoologia, CEP 78060-900, Cuiaba MT, Brazil 7INPA Núcleo de Pesquisas de Roraima (INPA/NPRR), Rua Coronel Pinto 315 – Centro, 69301-970, Boa Vista, RR, Brazil 8Corresponding author. E-mail: [email protected] Abstract We describe two new species of Anomaloglossus from Roraima State, Brazil, that are likely endemic to single mountains currently isolated among lowland forest and savanna ecosystems. The first species, Anomaloglossus tepequem sp. -

For Review Only
Page 63 of 123 Evolution Moen et al. 1 1 2 3 4 5 Appendix S1: Supplementary data 6 7 Table S1 . Estimates of local species composition at 39 sites in Middle America based on data summarized by Duellman 8 9 10 (2001). Locality numbers correspond to Table 2. References for body size and larval habitat data are found in Table S2. 11 12 Locality and elevation Body Larval Subclade within Middle Species present Hylid clade 13 (country, state, specific location)For Reviewsize Only habitat American clade 14 15 16 1) Mexico, Sonora, Alamos; 597 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 17 Smilisca baudinii 76.0 pond Middle American Smilisca clade 18 Smilisca fodiens 62.6 pond Middle American Smilisca clade 19 20 21 2) Mexico, Sinaloa, Mazatlan; 9 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 22 Smilisca baudinii 76.0 pond Middle American Smilisca clade 23 Smilisca fodiens 62.6 pond Middle American Smilisca clade 24 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 25 Diaglena spatulata 85.9 pond Middle American Smilisca clade 26 27 28 3) Mexico, Durango, El Salto; 2603 Hyla eximia 35.0 pond Middle American Hyla 29 m 30 31 32 4) Mexico, Jalisco, Chamela; 11 m Dendropsophus sartori 26.0 pond Dendropsophus 33 Exerodonta smaragdina 26.0 stream Middle American Plectrohyla clade 34 Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 35 Smilisca baudinii 76.0 pond Middle American Smilisca clade 36 Smilisca fodiens 62.6 pond Middle American Smilisca clade 37 38 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 39 Diaglena spatulata 85.9 pond Middle American Smilisca clade 40 Trachycephalus venulosus 101.0 pond Lophiohylini 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 Evolution Page 64 of 123 Moen et al. -

Species Diversity and Conservation Status of Amphibians in Madre De Dios, Southern Peru
Herpetological Conservation and Biology 4(1):14-29 Submitted: 18 December 2007; Accepted: 4 August 2008 SPECIES DIVERSITY AND CONSERVATION STATUS OF AMPHIBIANS IN MADRE DE DIOS, SOUTHERN PERU 1,2 3 4,5 RUDOLF VON MAY , KAREN SIU-TING , JENNIFER M. JACOBS , MARGARITA MEDINA- 3 6 3,7 1 MÜLLER , GIUSEPPE GAGLIARDI , LILY O. RODRÍGUEZ , AND MAUREEN A. DONNELLY 1 Department of Biological Sciences, Florida International University, 11200 SW 8th Street, OE-167, Miami, Florida 33199, USA 2 Corresponding author, e-mail: [email protected] 3 Departamento de Herpetología, Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Avenida Arenales 1256, Lima 11, Perú 4 Department of Biology, San Francisco State University, 1600 Holloway Avenue, San Francisco, California 94132, USA 5 Department of Entomology, California Academy of Sciences, 55 Music Concourse Drive, San Francisco, California 94118, USA 6 Departamento de Herpetología, Museo de Zoología de la Universidad Nacional de la Amazonía Peruana, Pebas 5ta cuadra, Iquitos, Perú 7 Programa de Desarrollo Rural Sostenible, Cooperación Técnica Alemana – GTZ, Calle Diecisiete 355, Lima 27, Perú ABSTRACT.—This study focuses on amphibian species diversity in the lowland Amazonian rainforest of southern Peru, and on the importance of protected and non-protected areas for maintaining amphibian assemblages in this region. We compared species lists from nine sites in the Madre de Dios region, five of which are in nationally recognized protected areas and four are outside the country’s protected area system. Los Amigos, occurring outside the protected area system, is the most species-rich locality included in our comparison. -

Etar a Área De Distribuição Geográfica De Anfíbios Na Amazônia
Universidade Federal do Amapá Pró-Reitoria de Pesquisa e Pós-Graduação Programa de Pós-Graduação em Biodiversidade Tropical Mestrado e Doutorado UNIFAP / EMBRAPA-AP / IEPA / CI-Brasil YURI BRENO DA SILVA E SILVA COMO A EXPANSÃO DE HIDRELÉTRICAS, PERDA FLORESTAL E MUDANÇAS CLIMÁTICAS AMEAÇAM A ÁREA DE DISTRIBUIÇÃO DE ANFÍBIOS NA AMAZÔNIA BRASILEIRA MACAPÁ, AP 2017 YURI BRENO DA SILVA E SILVA COMO A EXPANSÃO DE HIDRE LÉTRICAS, PERDA FLORESTAL E MUDANÇAS CLIMÁTICAS AMEAÇAM A ÁREA DE DISTRIBUIÇÃO DE ANFÍBIOS NA AMAZÔNIA BRASILEIRA Dissertação apresentada ao Programa de Pós-Graduação em Biodiversidade Tropical (PPGBIO) da Universidade Federal do Amapá, como requisito parcial à obtenção do título de Mestre em Biodiversidade Tropical. Orientador: Dra. Fernanda Michalski Co-Orientador: Dr. Rafael Loyola MACAPÁ, AP 2017 YURI BRENO DA SILVA E SILVA COMO A EXPANSÃO DE HIDRELÉTRICAS, PERDA FLORESTAL E MUDANÇAS CLIMÁTICAS AMEAÇAM A ÁREA DE DISTRIBUIÇÃO DE ANFÍBIOS NA AMAZÔNIA BRASILEIRA _________________________________________ Dra. Fernanda Michalski Universidade Federal do Amapá (UNIFAP) _________________________________________ Dr. Rafael Loyola Universidade Federal de Goiás (UFG) ____________________________________________ Alexandro Cezar Florentino Universidade Federal do Amapá (UNIFAP) ____________________________________________ Admilson Moreira Torres Instituto de Pesquisas Científicas e Tecnológicas do Estado do Amapá (IEPA) Aprovada em de de , Macapá, AP, Brasil À minha família, meus amigos, meu amor e ao meu pequeno Sebastião. AGRADECIMENTOS Agradeço a CAPES pela conceção de uma bolsa durante os dois anos de mestrado, ao Programa de Pós-Graduação em Biodiversidade Tropical (PPGBio) pelo apoio logístico durante a pesquisa realizada. Obrigado aos professores do PPGBio por todo o conhecimento compartilhado. Agradeço aos Doutores, membros da banca avaliadora, pelas críticas e contribuições construtivas ao trabalho. -

Amphibia: Anura: Aromobatidae:Mannophryne
Artículo original / Original article HERPETOTROPICOS 2006 Vol. 3(1):51-57 Copyright © 2007 Univ. Los Andes VARGAS GALARCE and LA MARCA - A NEW SPECIES OF COLLAREDPrinted FROG in Venezuela. All rights reserved51 ISSN 1690-7930 A NEW SPECIES OF COLLARED FROG (AMPHIBIA: ANURA: AROMOBATIDAE: MANNOPHRYNE) FROM THE ANDES OF TRUJILLO STATE, VENEZUELA JÉSSICA Y. VARGAS GALARCE1,2 AND ENRIQUE LA MARCA2,3 1 Departamento de Biología, Facultad de Ciencias, Universidad de Los Andes, Mérida, Venezuela. 2 Laboratorio de Biogeografía, Escuela de Geografía, Facultad de Ciencias Forestales y Ambientales, Universidad de Los Andes, Mérida, Venezuela. Abstract: We describe a new species of Mannophryne frog, coming from Trujillo State, in the Venezuelan Andes, which constitutes the first species of the genus described from that political unit. This new taxon is distinguished from its congeners by the following combination of characters: small size (SVL males 20.1-23.1 mm, females 23.3-27.5 mm); tympanum about ½ the horizontal length of eye; first finger almost equal, or slightly shorter than second; fingers with thick lateral fringes; tarsal fold conspicuous; foot-web formula: I1.0–0.5II1.0–1.0III1.5–0.5IV0.5–2.0V; toes with lateral flaps; short oblique pale inguinal band; collar moderately wide, solid, with small pale blotches; ventrolateral band absent. We provide data on coloration, in life and in preservative, of specimens of the type series, as well as a description of the larvae and ecological data for the species. Key words: Mannophryne, Amphibia, Anura, Venezuela, Andes, taxonomy, natural history, conservation. Resumen: J.Y. Vargas Galarce y E. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -
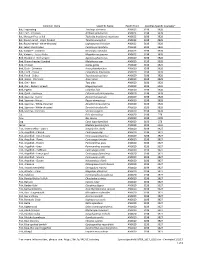
*For More Information, Please See
Common Name Scientific Name Health Point Specifies-Specific Course(s)* Bat, Frog-eating Trachops cirrhosus AN0023 3198 3928 Bat, Fruit - Jamaican Artibeus jamaicensis AN0023 3198 3928 Bat, Mexican Free-tailed Tadarida brasiliensis mexicana AN0023 3198 3928 Bat, Round-eared - stripe-headed Tonatia saurophila AN0023 3198 3928 Bat, Round-eared - white-throated Lophostoma silvicolum AN0023 3198 3928 Bat, Seba's short-tailed Carollia perspicillata AN0023 3198 3928 Bat, Vampire - Common Desmodus rotundus AN0023 3198 3928 Bat, Vampire - Lesser False Megaderma spasma AN0023 3198 3928 Bird, Blackbird - Red-winged Agelaius phoeniceus AN0020 3198 3928 Bird, Brown-headed Cowbird Molothurus ater AN0020 3198 3928 Bird, Chicken Gallus gallus AN0020 3198 3529 Bird, Duck - Domestic Anas platyrhynchos AN0020 3198 3928 Bird, Finch - House Carpodacus mexicanus AN0020 3198 3928 Bird, Finch - Zebra Taeniopygia guttata AN0020 3198 3928 Bird, Goose - Domestic Anser anser AN0020 3198 3928 Bird, Owl - Barn Tyto alba AN0020 3198 3928 Bird, Owl - Eastern Screech Megascops asio AN0020 3198 3928 Bird, Pigeon Columba livia AN0020 3198 3928 Bird, Quail - Japanese Coturnix coturnix japonica AN0020 3198 3928 Bird, Sparrow - Harris' Zonotrichia querula AN0020 3198 3928 Bird, Sparrow - House Passer domesticus AN0020 3198 3928 Bird, Sparrow - White-crowned Zonotrichia leucophrys AN0020 3198 3928 Bird, Sparrow - White-throated Zonotrichia albicollis AN0020 3198 3928 Bird, Starling - Common Sturnus vulgaris AN0020 3198 3928 Cat Felis domesticus AN0020 3198 279 Cow Bos taurus