Structure, Physical Characteristics, and Com- Position of the Pericarp and Integument of Johnson Grass Seed in Relation to Its Physiology 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Morphological, Anatomical, and Taxonomic Studies in Anomochloa and Streptochaeta (Poaceae: Bambusoideae)
SMITHSONIAN CONTRIBUTIONS TO BOTANY NUMBER 68 Morphological, Anatomical, and Taxonomic Studies in Anomochloa and Streptochaeta (Poaceae: Bambusoideae) Emmet J. Judziewicz and Thomas R. Soderstrom SMITHSONIAN INSTITUTION PRESS Washington, D.C. 1989 ABSTRACT Judziewicz, Emmet J., and Thomas R. Soderstrom. Morphological, Anatomical, and Taxonomic Studies in Anomochloa and Streptochaeta (Poaceae: Bambusoideae). Smithsonian Contributions to Botany, number 68,52 pages, 24 figures, 1 table, 1989.-Although resembling the core group of the bambusoid grasses in many features of leaf anatomy, the Neotropical rainforest grass genera Anomochloa and Streptochaeta share characters that are unusual in the subfamily: lack of ligules, exceptionally long microhairs with an unusual morphology, a distinctive leaf blade midrib structure, and 5-nerved coleoptiles. Both genera also possess inflorescences that are difficult to interpret in conventional agrostological terms. Anomochloa is monotypic, and A. marantoidea, described in 1851 by Adolphe Brongniart from cultivated material of uncertain provenance, was rediscovered in 1976 in the wet forests of coastal Bahia, Brazil. The inflorescence terminates in a spikelet and bears along its rachis several scorpioid cyme-like partial inflorescences. Each axis of a partial inflorescence is subtended by a keeled bract and bears as its first appendages two tiny, unvascularized bracteoles attached at slightly different levels. The spikelets are composed of an axis that bears two bracts and terminates in a flower. The lower, chlorophyllous, deciduous spikelet bract is separated from the coriaceous, persistent, corniculate upper bract by a cylindrical, indurate internode. The flower consists of a low membrane surmounted by a dense ring of brown cilia (perigonate annulus) surrounding the andrecium of four stamens, and an ovary bearing a single hispid stigma. -

Plant Anatomy Lab 13 – Seeds and Fruits
Plant Anatomy Lab 13 – Seeds and Fruits In this (final) lab, you will be observing the structure of seeds of gymnosperms and angiosperms and the fruits of angiosperms. Much of the work will be done with a dissecting microscope, but a few prepared slides will also be used. A set of photocopied images from the plant anatomy atlas will be available as a handout. You can use the handout to help you identify the various structures we will be looking at in seeds and fruits. Also, a fruit key is available as a separate handout. Remember that we will be considering only a small fraction of the structural diversity present among seeds of gymnosperms and the seeds and fruits of angiosperms. Seeds Gymnosperms Obtain a prepared slide of an immature pine ovule and of a mature pine ovule. You will be able to tell them apart from the following observations. • Looking at the immature ovule, you will see a megagametophyte with one or more archegonia at the end near the micropyle. The egg inside may or may not have been fertilized. • Also find the nucellus and integument tissues. Next look at a slide of a mature ovule. Instead of the megagametophyte, you will find a developing embryo. As part of this embryo, find the • cotyledons, • the radicle (embryonic root), and the • shoot apical meristem. Depending on its age, you may also notice procambial strands running between the embryonic root and the shoot apex. Points to consider: We might say that gymnosperms and angiosperms have seeds but only angiosperms have fruits - Why is that? Why don’t we consider the seed cone of a pine tree a fruit? Angiosperms Dicot Obtain a bean pod. -

KEY to FRUIT TYPES 1A. Fruit Derived from Several Ovaries of One Or More Flowers 2A. Fruit Arising from the Several Ovaries of A
KEY to FRUIT TYPES 1a. Fruit derived from several ovaries of one or more flowers 2a. Fruit arising from the several ovaries of as many flowers (examples: pineapple, mulberry) MULTIPLE FRUIT 2b. Fruit arising from the coalescence of several ripened ovaries of one flower (example: raspberry, blackberry) AGGREGATE FRUIT 1b. Fruit derived from a single ovary (simple or compound) 3a. Fruit fleshy or juicy when ripe 4a. Ovary wall of fruit (or pericarp) entirely or in part fleshy 5a. Fruit indehiscent 6a. Ovary wall entirely fleshy (examples: tomato, cranberry, grape, currant, banana, melon [pepo], and citrus fruit [hesperidium]) BERRY 6b. Ovary wall of three distinct layers, the inner one bony (endocarp), the middle fleshy (mesocarp), and the outer "skin- like" (exocarp) (examples: peach, plum, cherry) DRUPE 5b. Fruit dehiscent 7a. Fruit derived from one carpel FOLLICLE 7b. Fruit derived from a compound gynoecium CAPSULE 4b. Ovary wall (e.g., the outer layer of an apple 'core') of fruit papery, surrounded by a fleshy material that represents the coalescent parts of the stamens, petals, sepals, and (some believe) receptacle (examples: apple, pear, quince) POME 3b. Fruit typically dry and usually hardened when ripe 8a. Fruit indehiscent (does not open or dehisce when mature), generally with one seed 9a. Ovary wall of varying thickness, usually not bony 10a. Fruit not winged (examples: buttercup, 'seeds' of strawberry, sunflower family, sedges, grasses [ovary wall adherent to and surrounding seed, may be called caryopsis or grain]) ACHENE 10b. Fruit winged (examples: elm, tulip tree) SAMARA 9b. Ovary wall hardened and bony 11a. Fruit usually > 5mm long (examples: oak, chestnut, hazelnut) NUT 11b. -

Caryopsis Student Newsletter – Winter/Spring 2020 Greetings to All Student Members of the Cereals & Grains Association! with a New Year Comes New Beginnings
Caryopsis Student Newsletter – Winter/Spring 2020 Greetings to all student members of the Cereals & Grains Association! With a new year comes new beginnings. Our organization officially changed names from AACC International to the Cereals & Grains Association this past fall 2019. With this new name also comes expanded opportunities that build upon the previous community and resources that were already established. Not only are there changes in our overall organization, but there are also new and exciting changes ahead for our Cereals & Grains Student Association. I am most honored to serve as this year’s Chair of the Student Association, heading a passionate, highly skilled, and ambitious group of student leaders. We will be keeping you all updated as the year progresses. I look forward to our continued success! As for a little about myself, I am a PhD candidate under the advisement of Dr. Bruce Hamaker in the Department of Food Science at Purdue University. Thus far my research has focused on investigating the slow digestion and satiety properties of pearl millet grown in sub-Saharan Africa as well as exploring the implications of starch fine structural features on digestibility and texture applications through collaborative projects. Ultimately, my work will help identify characteristics of glycemic carbohydrates that impart a slow digestion property and elucidate how they can be leveraged to design foods with targeted physiological outcomes. Before graduate school, I received dual bachelor’s degrees in Food and Nutrition Science as well as Spanish from St. Catherine University in St. Paul, MN. In my free time, I enjoy being outdoors, music, and traveling. -

Cereals & Legumes
BIOL 221 – Concepts of Botany Fall 2010 Important Angiosperm Foods: Cereals & Legumes Although there are several major lineages of plants, our society relies essentially on just one of these, the angiosperms, for food. Today you will acquaint yourselves with some of the two major group angiospermous food plants and their products. Before Coming to Lab: Read sections A (intro), A1, A2, A3, B (intro), B2, and B3. Then answer the following questions. 1. What is a cereal and which lab page did you find the definition? 2. What is a pulse and on which page did you find that definition.? 3. What is the technical definition of a legume fruit and on which page...? 4. Which, the cereals or the pulses, are the most important to humanity in terms of total calories consumed? 5. Which had more abundant starch: cereals or pulses? 6. Which has more abundant protein: cereals or pulses? Concepts of Botany, page 1 of 15 Concepts of Botany, page 2 of 15 A. Cereal Morphology & Anatomy Cereals are grasses (family Poaceae) cultivated for their edible grains. The grain of a grass is a particular type of fruit unique to the family called a “caryopsis.” Cereals include wheat, maize (aka corn in the USA), rye, barley, oats, and rice among others. They are generally the most important food source for humanity in terms of the total calories they contribute to your diet, either directly or indirectly. Table 1. Cereals discussed in this lab manual and their native ranges. Species (common name) Native Range, Origin of Domestication Avena sativa (oats) Europe Hordeum vulgare (barley) SW Asia Oryza sativa (common rice) SE Asia Triticum aestivum (bread wheat) SW Asia Zea mays (corn, maize) Mexico Zizania aquatica (wild rice) North America 1. -

Flowers, Inflorescences & Fruits 2020
Australian Plants Society NORTH SHORE GROUP Ku-ring-gai Wildflower Garden Flowers, Inflorescences and Fruits FLOWERS In common usage the word ‘flower’ is used for both a single flower and a number of flowers grouped together, for example a Banksia spike. Closer examination shows it is made up of single flowers, all with a similar structure. A flower is the sexual reproductive shoot of a plant, consisting of a receptacle that bears the sepals, petals, stamens and carpels – the four basic parts of a flower. Broadly speaking, the parts are in concentric rings. Sepal: Makes up the outer ring, usually green and leaf-like, and in the bud stage encloses and protects the other flower parts. Collectively known as the calyx. Sepals could be free, wholly or partly united, they could fall early or remain as part of the fruit. Petal: Makes up the next inner ring, usually conspicuous, brightly coloured, to attract pollinators. Collectively known as the corolla. They could also be free, part or fully united giving rise to variety of types. Tepal: A free segment of a perianth not recognized as a petal or a sepal. Perianth: Usually consisting of a whorl of sepals and/or a whorl of petals, or two whorls of tepals. Pedicel: (stalk) of a flower, if not present the flower is sessile. Female part of the flower Gynoecium: the carpel (if solitary) or carpels of a flower. Carpel: A unit of the female organ of the flower, with an ovary bearing one or more ovules (female cells), usually a style (stalk), joining the ovary and a pollen receptive stigma of various shapes and size. -
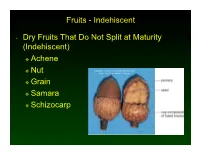
Indehiscent • Dry Fruits That Do Not Split at Maturity
Fruits - Indehiscent • Dry Fruits That Do Not Split at Maturity (Indehiscent) Achene Nut Copyright © McGraw-Hill Companies Permission Required for Reproduction or Display Grain Samara Schizocarp Fruits - Indehiscent • Achene- a single-seeded fruit in which the seed is attached to the pericarp only at its base • The pericarp, the husk, is easily separated from the seed. • Ex. Sunflower, dandelion Fruits - Indehiscent • Nut- achene variation- one seeded, dry fruit with a hard, thick pericarp; develops with a cup or cluster of bracts at base • Ex- acorn, chestnut, hazelnut Fruits - Indehiscent • Grain (caryopsis)- a dry fruit in which the pericarp is tightly fused to the seed • Ex- corn, rice, wheat Fruits - Indehiscent • Samara- a dry fruit whose pericarp extends around the seed in the form of a wing • Ex. Maple, ash Fruits - Indehiscent • Schizocarp- a twin fruit that separates at maturity into two one-seeded fruitlets • Ex- parsley, carrot, dill Fruits • Aggregate Fruits- derived from a single flower with several to many pistils • Individual pistils mature as a clustered unit on a single receptacle • Ex- raspberries, strawberries Fruit • Multiple Fruit- derived from several to many individual flowers in a single inflorescence • Ex. Pineapple, fig, Osage orange, mulberries Fruit and Seed Dispersal • Wind Dispersal Small and Lightweight seeds. May have attachments like wings or hairs to help give them lift. Example- maple, ash, dandelion • Animal Dispersal Seeds can pass through an animal’s digestive tract. Some fruits and seeds have spines or thorns that catch in fur or feathers. Oils attract ants. Fruit and Seed Dispersal • Water Dispersal Some fruits contain trapped air. -

Dataset Development of Fruit Types and Seed Dispersal Modes of Plants in Five Communities in Shilin Geopark, Yunnan, China
Journal of Global Change Data & Discovery. 2019, 3(2): 187-193 ©2019 GCdataPR DOI:10.3974/geodp.2019.02.10 Global Change Research Data Publishing & Repository www.geodoi.ac.cn Dataset Development of Fruit Types and Seed Dispersal Modes of Plants in Five Communities in Shilin Geopark, Yunnan, China Yu, X. Y.1,2 Li, Y. H.2* 1. School of Tourism and Resource Environment, Qiannan Normal University for Nationalities, Duyun 558000, Guizhou, China; 2. School of Tourism and Geography Science, Yunnan Normal University, Kunming 650500, China Abstract: The sexual propagule sources for vegetation regeneration are characterized by fruit type and seed dispersal mode. These sexual propagule sources play an important role in the con- trol of degraded ecosystems, including ecosystems affected by karst rocky desertification. To study the sexual propagule sources in Shilin Geopark, Yunnan, China, the vascular plant lists from five communities (obtained using the quadrat method from August to September 2003) were investi- gated. The five communities were zonal forest, secondary forest, shrubland, shrub tussock, and Pinus yunnanensis plantation forest. The fruit type of each species was determined based on “Flora of China”. The seed dispersal mode of each species was determined based on the pub- lished literature, the Kew Seed Information Database (http://data.kew.org/sid/), and the fruit and seed morphological traits. In total, 16 fruit types (including spore) were observed for the 282 vas- cular plant species in the communities in Shilin Geopark. Achenes (17.02%), capsules (16.67%), berries (14.18%), and drupes (12.41%) were the most common fruit types. The dominant seed dispersal modes were zoochory (47.87%) and anemochory (33.69%). -

Lecture 27-28. Fruits Topics Formation of Fruits Basic Fruit Types Fruit Types
Lecture 27-28. Fruits Topics • Formation of fruits • Basic Fruit Types Formation of fruits Basic Fruit Types • The two principal Fruit Types are Fleshy & Dry – Caution: A Legume is a dry fruit. We eat unripe legumes like Snow Peas and Green Beans. We might classify the latter as fleshy fruits but they are dry at maturity!! • Dry Fruits are either Dehiscent or Indehiscent. – Dehiscent Fruits open at maturity while indehiscent Fruits do not! • Fruits may be Simple or Accessory. – Simple Fruits are Mature Carpels. – Accessory Fruits include the Carpels & other tissues. • The latter may be other Floral Organs or the Receptacle. • Aggregate vs multiple fruits – Aggregate Fruits contain Many Simple Carpels from ONE FLOWER – Multiple Fruits contain the Fruits of MANY FLOWERS. Fruit Types 1 Fruit Types: simple fruits Simple Fruits • Simple Fruits – Simple fruits are derived from • Fleshy single or several united carpels. - Drupe: peach – Legumes are fruits that split along two sides when mature. - Berry: grape • Dehiscent - Split open - Pome: apple • Indehiscent - Fail to split open •Dry – Dehiscent • Follicle: peony • Legume: bean • Capsule: poppy – Indehiscent • Achene: sunflower • Nut: hazelnut • Grain: rice Simple Fruits – Dispersal • Many seeds are dispersed by wind. – Woolly hairs, plumes, wings – Fleshy fruits - Attract animals • Berry. Fleshy fruit, with succulent and provide them with food. • Peaches, cherries, tomatoes pericarp, as in Vitis. – Accessory fruit - Bulk of fruit is not from ovary, but from • Drupe. A fleshy fruit with a stony receptacle. endocarp, as in Prunus. • Apples • Drupelet. A small drupe, as in Rubus. Berry – from compound ovary with many seeds Drupe – from simple ovary with one seed and soft “skin” • have a fleshy or leathery Grapes (Vitis) • like Berries but they Exocarp, Mesocarp and have Stony Endocarps Endocarp. -
BBCH-Scale Hack Et Al., 1992
Growth stages of mono-and dicotyledonous plants BBCH Monograph 2. Edition, 2001 Edited by Uwe Meier Federal Biological Research Centre for Agriculture and Forestry The code has been jointly by – German Federal Biological Research Centre for Agriculture and Forestry (BBA) – German Federal Office of Plant Varieties (BSA) – German Agrochemical Association (IVA) – Institute for Vegetables and Ornamentals in Grossbeeren/Erfurt, Germany Members of the BBCH working group H. Bleiholder und Frau E. P. D. Lancashire Weber Bayer plc. BASF AG Eastern Way Landwirtschaftliche Ver- Bury St. Edmunds suchsstation Suffolk IP 32 7 AH, UK Carl-Bosch-Strasse 64 D-67117 Limburgerhof Frau L. Buhr Biologische Bundesanstalt C. Feller für Land- und Forstwirtschaft Institut für Gemüse & Zier- Stahnsdorfer Damm 81 pflanzenbau D-14532 Kleinmachnow Theodor-Echtermeyer-Weg 1 D-14979 Grossbeeren H. Hack Industrieverband Agrar (IVA) M. Hess und H. Wicke Theodor-Storm-Weg 2 Aventis D-51519 Odenthal D-65926 Frankfurt/Main Frau R. Klose U. Meier Bundessortenamt Biologische Bundesanstalt Osterfelddamm 80 für Land- und Forstwirtschaft D-30604 Hannover Messeweg 11/12 D-38104 Braunschweig R. Stauss Ministerium für ländliche T. van den Boom Räume, Landwirtschaft, Bayer AG Ernährung und Tourismus Landwirtschaftszentrum des Landes Schleswig- Monheim Holstein Alfred-Nobel-Strasse 50 Düsternbrooker Weg 104 D-51368 Leverkusen- D-24105 Kiel Bayerwerk General Scale Cereals, Rice, Maize Oilseed rape, Faba bean, Sunflower Beta beets Potato Fruits Citrus, Olive, Coffee, Banana Grapevine Soybean, Cotton, Peanuts Hop Vegetable crops I Vegetable crops II Weeds BBCH-Publications Foreword As all branches of science, the individual disciplines in agricultural plant research also work more closely together, and, in addition, have become more international. -
Bio 320 - Ethnobotany April 16, 2015 Basic Plant Identification
1 Bio 320 - Ethnobotany April 16, 2015 Basic Plant Identification I. Terminology and vocabulary It is important that we develop a good working knowledge of botanical terminology and vocabulary - thus we will spend today working on developing that knowledge. This handout lists many terms that are necessary for identifying plants via a key. Don’t worry about memorizing them all, but you should feel comfortable with them. I am also giving you a copy of a key to families of plants native to Illinois. You should use that key to work some through the demonstration plants. Also be sure to examine the herbarium sheets for examples of interesting local plants. Handle the herbarium sheets very carefully. They are fragile. A. Habit - extremely important in identifying plants grossly - trees, shrubs, herbs woody - trees - large, 1 main stem - shrubs - smaller, several main stems herbs - no well developed woody layer suffrutescent - only slightly woody, can be large - often upper parts of stems are herbaceous suffruticose - diminutive shrub - very woody habit - stems acaulescent - no stem - a dandelion caulescent - have stem cespitose - in little tufts or dense clumps - used for small plants growing in turf B. Plant parts Vegetative parts - roots, stems, leaves Reproductive parts - flowers and fruits Roots - often ignored in identifying plants, though can be important taproot - more or less fleshy, goes straight into ground - many dicots fibrous roots - bushy, thin fibers - all monocots Stems can also be important in identification - herbaceous vs. -

Fruit Classification
Name______________________ Lab: Fruit Types and Classification of Fruits What is a fruit? Botanically speaking, a fruit is a ripened ovary with seeds and any other structures that enclose it at maturity. This definition of a fruit means that many ‘vegetables’ are fruits (squash, tomatoes, beans, corn) and many ‘grains’ are also fruit (rice, wheat, etc.). Remember that the ovary is part of the pistil, or female part of the flower. When pollen from the stamen reaches the pistil and travels down the style into the ovary, it can fertilize ovules and the seeds and fruit begin to develop. While the seeds develop from the ovules, the ovary tissue undergoes a series of complex changes that results in the development of the fruit. As the ovary develops into a fruit, its wall often thickens and develops three layers: · Exocarp- the outermost layer (often like a skin or peel). · Mesocarp- the middle layer (often fleshy), varies in thickness · Endocarp- the innermost layer (often hard, stony or papery) Together, these layers are called the pericarp. These layers are most visible in “fleshy” fruits: In this lab, you will learn about several common fruit types. I. Fleshy fruits. A. Drupe. A fleshy fruit formed from one ovary with a single stony seed. Examine the Avocado and answer the following questions: What part of the ovary wall is the rough skin of the avocado? What part of the ovary wall forms the fleshy part that we eat? What part of the fruit forms the stone or pit? Name another common drupe that we eat. B.